Quelet reaction
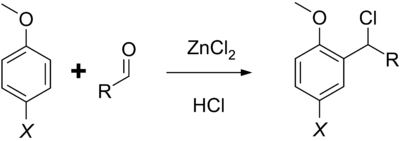
The Quelet reaction (also called the Blanc–Quelet reaction) is an organic coupling reaction in which a phenolic ether reacts with an aliphatic aldehyde to generate an α-chloroalkyl derivative.[1] The reaction is named after its creator R. Quelet, who first reported the reaction in 1932,[2] and is similar to the Blanc chloromethylation process.
The reaction proceeds under strong acid catalysis using HCl; zinc(II) chloride may be used as a catalyst in instances where the ether is deactivated.[3] The reaction primarily yields para-substituted products; however it can also product ortho-substituted compounds if the para site is blocked.
See also
References
- ↑ Wang, Zerong (2009). "517: Quelet Reaction". Comprehensive organic name reactions and reagents. Hoboken, N.J.: John Wiley. pp. 2290–2292. ISBN 9780470638859.
- ↑ R. Quelet (1932). "preparation d'un derive chloro-methyl du para-bromo-anisol (methoxy-2 bromo-2 α-chlorotoluene).". Compt. Rend. (in French) (T195): 155.
- ↑ Denmark], [editor-in-chief, Scott E. (2006). "1:3 Chloromethylation of Aromatic Compounds". Organic reactions. Hoboken, N.J.: Wiley. pp. 63–90. ISBN 9780471264187.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.