Pupillometry
Pupillometry, the measurement of pupil size and reactivity, is a key part of the clinical neurological exam for patients with a wide variety of neurological injuries. It is also used in psychology.[1][2]
Pupillometry in critical care
For more than 100 years, clinicians have evaluated the pupils of patients with suspected or known brain injury or impaired consciousness to monitor neurological status and trends, checking for pupil size and reactivity to light.[3] In fact, before the advent of electricity, doctors checked a patient’s reaction to light using a candle.
Today, clinicians routinely evaluate pupils as a component of the neurological examination and monitoring of critically ill patients, including patients with traumatic brain injury and stroke.[4][5][6]
Patient care and outcome
Numerous studies have shown the importance of pupil evaluation in the clinical setting, and pupillary information is used extensively in patient management and as an indication for possible medical intervention.
Patients who undergo prompt intervention after a new finding of pupil abnormality have a better chance of recovery.[7]
Alterations of the pupil light reflex, size of the pupil, and anisocoria (unequal pupils) are correlated with outcomes of patients with traumatic brain injury.[2][8][9][10][11][12][13][14][15][16][17] Blood flow imaging has shown that pupil changes are highly correlated with brainstem oxygenation and perfusion,[16][15][18] and anisocoria can be an indicator of a pathological process or neurological dysfunction.[15][19][20]
Investigators have used pupil size and reactivity as fundamental parameters of outcome predictive models in conjunction with other clinical information such as age, mechanism of injury, and Glasgow Coma Scale,[18][21][22] and have correlated the models with the presence and location of intracranial mass lesions.[8]
The National Institutes of Health Stroke Scale (NIHSS) uses pupillary response as a systematic assessment tool to provide a quantitative measure of stroke-related neurologic deficit and to evaluate acuity of stroke patients, determine appropriate treatment, and predict patient outcome.[23]

Manual vs. automated pupil assessment
Traditionally, pupil measurements have been performed in a subjective manner by using a penlight or flashlight to manually evaluate pupil reactivity and using a pupil gauge to estimate pupil size. However, manual pupillary assessment is subject to significant inaccuracies and inconsistencies. Studies have shown inter-examiner disagreement in the manual evaluation of pupillary reaction to be as high as 39 percent.[1][2][4][5][24][25][26][27]
Automated pupillometry involves the use of a pupilometer, a portable, handheld infrared device that provides a reliable and objective measurement of pupillary size, symmetry, and reactivity through measurement of the pupil light reflex. A numeric scale allows a more rigorous interpretation and classification of the pupil response.
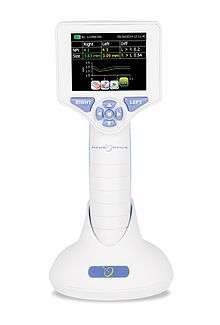
Automated pupillometry removes subjectivity from the pupillary evaluation, providing more accurate and trendable pupil data, and allowing earlier detection of changes for more timely patient treatment.
A recent study published in the American Journal of Critical Care revealed that critical care and neurosurgical nurses consistently underestimated pupil size, were unable to identify anisocoria, and incorrectly assessed pupil reactivity. It concluded that automated pupillometry is a necessary tool for accuracy and consistency, and that it might facilitate earlier detection of subtle pupil changes, allowing more effective and timely diagnostic and treatment interventions.[1]
In addition, a study from The University of Texas Southwestern Medical Center compared 2,329 manual pupillary exams performed simultaneously by two examiners (neurology and neurosurgery attending and resident physicians, staff nurses, and mid-level practitioners) under identical conditions and showed low inter-examiner reliability.[25][26]
Automated pupillometry in critical care nursing textbooks
The American Association of Critical-Care Nurses (AACN) Procedure Manual for High Acuity, Progressive and Critical Care, 7th Edition, and the American Association of Neuroscience Nurses (AANN) Core Curriculum for Neuroscience Nursing, 6th Edition, now include sections illustrating how use of a pupillometer removes subjectivity and allows pupillary reactivity to be trended in a consistent, objective, and quantifiable way. The AACN Procedure Manual, which was extensively reviewed by more than 100 experts in critical care nursing, is the authoritative reference for procedures performed in critical care settings, and the AANN curriculum is a comprehensive resource for practicing neuroscience nurses.
Pupillometry in psychology
Stimulants
Photographs
Hess and Polt (1960)[28] presented pictures of semi-naked adults and babies to adults (four men and two women). Pupils of both sexes dilated after seeing pictures of people of the opposite sex. In females, the difference in pupil size occurred also after seeing pictures of babies and mothers with babies. This examination showed that pupils react not only to the changes of intensity of light (pupillary light reflex) but also reflect arousal or emotions.
In 1965 Hess, Seltzer and Shlien[29] examined pupillary responses in heterosexual and homosexual males. Results showed a greater pupil dilation to pictures of the opposite sex for heterosexuals and to pictures of the same sex for homosexuals .
According to T.M. Simms (1967),[30] pupillary responses of males and females were greater when they were exposed to pictures of the opposite sex.[31] In another study, Nunnally and colleagues (1967)[32] found that seeing slides rated as 'very pleasant' was associated with greater pupil dilation as seeing slides rated as neutral or very unpleasant.
Infants showed greater pupil size when they saw pictures of faces than when they saw geometric shapes,[31][33][34] and greater dilation after seeing pictures of the infant's mother than pictures of a stranger.[33]
Cognitive load
Pupillary responses can reflect activation of the brain allocated to cognitive tasks. Greater pupil dilation is associated with increased processing in the brain.[35] Vacchiaco and colleagues (1968) found that pupillary responses were associated with visual exposure to words with high, neutral or low value. Presented low-value words were associated with dilation, and high-value words with constriction of a pupil.[36] In decision-making tasks dilation increased before the decision as a function of cognitive load.[37][38] In an experiment about short-term serial memory, students heard strings of words and were asked to repeat them. Greater pupil diameter was observed after the items were heard (depending on how many items were heard), and decreased after items were repeated.[39] The more difficult the task, the greater pupil diameter observed from the time preceding the solution [40] until the task was completed.[41]
Long-term memory
The pupil response reflects long-term memory processes both at encoding, predicting the success of memory formation[42] and at retrieval, reflecting different recognition outcomes.[43]
See also
References
- 1 2 3 Kerr, R (2016). "Underestimation of pupil size by critical care and neurosurgical nurses". American J of Critical Care. 25: 213–219.
- 1 2 3 Olson, D (2015). "The use of automated pupillometry in critical care". Critical Care Nursing Clinics North America. 28: 101–107.
- ↑ Loewenfeld, I. (1993). "The Pupil: Anatomy, Physiology, and Clinical Application". Ames: Iowa State University Press.
- 1 2 Meeker, M (2005). "Pupil examination: validity and clinical utility of an automated pupillometer". J Neurosci Nurs. 37: 34–40.
- 1 2 Wilson, S (1988). "Determining interrater reliability of nurses' assessments of pupillary size and reaction". J Neurosci Nurs. 20: 189–192.
- ↑ Chestnut, R (2000). "Management and Prognosis of Severe Traumatic Brain Injury. Part II: Early Indicators of Prognosis in Severe Traumatic Brain Injury". Brain Trauma Foundation, American Association of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care. 41.
- ↑ Clusmann, H (2001). "Fixed and dilated pupils after trauma, stroke, and previous intracranial surgery: management and outcome". J Neurol Neurosurg Psychiatry. 71: 175–181.
- 1 2 Braakman R, Gelpke G, Habbema J, Maas A, Minderhoud J. Systemic selection of prognostic features in patients with severe head injury. Neurosurgery. 1980;6:362–370
- ↑ Chen J, Gombart Z, Rogers S, Gardiner S, Cecil S, Bullock R. Pupillary reactivity as an early indicator of increased intracranial pressure: the introduction of the neurological pupil index. Surg Neurol Int. 2011;2:82.
- ↑ Chesnut R, Gautille T, Blunt B, Klauber M, Marshall L. The localizing value of asymmetry in pupillary size in severe head injury: relation to lesion type and location. Neurosurgery. 1994;34:840–845.
- ↑ Choi S, Narayan R, Anderson R, Ward J. Enhanced specificity of prognosis in severe head injury. J Neurosurg. 1988;69:381–385.
- ↑ Levin H, Gary H, Eisenberg H, et al. Neurobehavioral outcome 1 year after severe head injury. Experience of the Traumatic Coma Data Bank. J Neurosurg. 1990;73:699–709.
- ↑ Marshall L, Gautille T, Klauber M, et al. The outcome of severe closed head injury. J Neurosurg. 1991;75:28–36.
- ↑ Ritter A, Muizelaar J, Barnes T, et al. Brain stem blood flow, pupillary response, and outcome in patients with severe head injuries. Neurosurg. 1999;44(5):941-948.
- 1 2 3 Sakas D, Bullock M, Teasdale G. One-year outcome following craniotomy for traumatic hematoma in patients with fixed dilated pupils. J Neurosurg. 1995;82:961–965.
- 1 2 Taylor W, Chen J, Meltzer H, et al. Quantitative pupillometry, a new technology: normative data and preliminary observations in patients with acute head injury. J Neurosurg. 2003;98:205–213.
- ↑ Tien H, Cunha J, Wu S, et al. Do trauma patients with a Glasgow Coma Scale score of 3 and bilateral fixed and dilated pupils have any chance of survival? J Trauma. 2006;60:274–278.
- 1 2 Zhao D, Weil M, Tang W, Klouche K, Wann S. Pupil diameter and light reaction during cardiac arrest and resuscitation. Crit Care Med. 2001;4(29):825-828.
- ↑ Andrews B, Pitts L. Functional recovery after traumatic transtentorial herniation. Neurosurgery. 1991;29:227–231.
- ↑ Petridis A, Dörner L, Doukas A, Eifrig S, Barth H, Mehdorn M. Acute subdural hematoma in the elderly: clinical and CT factors influencing the surgical treatment decision. Cen Eur Neurosurg. 2009;70:73–78.
- ↑ Marmarou A, Lu J, Butcher I, et al. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: an IMPACT analysis. J Neurotrauma. 2007;24:270–280.
- ↑ Narayan R, Greenberg R, Miller J, et al. Improved confidence of outcome prediction in severe head injury: A comparative analysis of the clinical examination, multimodality evoked potentials, CT scanning, and intracranial pressure. J Neurosurg. 1981;54:751–762.
- ↑ NIH Stroke Scale (NIHSS). http://www.nihstrokescale.org/.
- ↑ Litvan I, Saposnik G, Mauriño J, et al. Pupillary diameter assessment: need for a graded scale. Neurology. 2000;54:530–531.
- 1 2 Olson D, Stutzman S, Saju C, Wilson M, Zhao W, Aiyagari V. Interrater reliability of pupillary assessments. Neurocritical Care. 2015.
- 1 2 Stutzman S, Olson D, Saju C, Wilson M, Aiyagari V. Interrater reliability of pupillar assessments among physicians and nurses. UT Southwestern Medical Center; 2014.
- ↑ Worthley L. The pupillary light reflex in the critically ill patient. Critical Care and Resuscitation. 2000;2(7).
- ↑ Hess E. H., Polt J. M. (1960) "Pupil size as related to interest value of visual stimuli" Science, 132, 349-350.
- ↑ Hess E. H., Seltzer A. L., Shlien J.M. (1965) "Pupil response of hetero- and homosexual males to pictures of men and women: A pilot study" Journal of Abnormal Psychology, 70, 165-168
- ↑ Simms T. M. (1967) "Pupillary response of male and female subjects to pupillary difference in male and female picture stimuli" Perception and Psychophysics, 2, 553-555.
- 1 2 Goldwater B. C. (1972) "Psychological significance of pupillary movements" Psychological Bulletin 77(5):340-55.
- ↑ Nunally J. C., Knott P. D., Duchnowski A., Parker R. (1967) "Pupillary response as a general measure of activation." Perception and Psychophysics, 2, 149-155
- 1 2 Fitzgerald H. E. (1968), "Autonomic pupillary reflex activity during early infancy and its relation to social and nonsocial visual stimuli" Journal of Experimental Child Psychology, 6, 470-482
- ↑ Fitzgerald H. E., Lintz L. M., Brackbill Y., Adams G. (1967), "Time perception and conditioning an autonomic response in human infants" Perceptual and Motor Skills, 24, 479-486
- ↑ Granholm E., Steinhauer S. R. (2004) "Pupillometric measures of cognitive and emotional processes" International Journal of Psychophysiology, Vol 52(1), 1-6.
- ↑ Vacchiano R. B., Strauss P. S., Ryan S., Hochman L. (1968) "Pupillary response to value-lined words" Perceptual and Motor Skills, 27, 207-210.
- ↑ Simpson H. M., Hale S. M., (1969) "Pupillary Changes During a Decision-Making Task" Perceptual and Motor Skills, 29, 495-498.
- ↑ Kahneman D., Beatty J. (1967) "Pupillary Response in a Pitch Discrimination Task" Perception and Psychophysics, 2, 101-105.
- ↑ Kahneman D., Beatty J. (1966) "Pupil Diameter and Load on Memory" Science, 154, 1583-1585.
- ↑ Hess E. H., Polt J. H. (1964) "Pupil Size in Relation to Mental Activity During Simple Problem Solving" Science, 143, 1190-1192
- ↑ Bradshaw J. L. (1968), "Pupil size and problem solving" Quarterly Journal of Experimental Psychology, Volume 20, Issue 2, 116-122.
- ↑ Kafkas, A., & Montaldi, D. (2011). Recognition memory strength is predicted by pupillary responses at encoding while fixation patterns distinguish recollection from familiarity. The Quarterly Journal of Experimental Psychology, 64(10), 1971–1989. http://www.tandfonline.com/doi/abs/10.1080/17470218.2011.588335
- ↑ Kafkas, A., & Montaldi, D. (2012). Familiarity and recollection produce distinct eye movement and pupil and medial temporal lobe responses when memory strength is matched. Neuropsychologia, 50(13), 3080–93. doi:10.1016/j.neuropsychologia.2012.08.001
Further reading
- Tryon, W. W. (1975), "Pupillometry: A Survey of Sources of Variation" Psychophysiology, 12: 90–93.
- Verney, S. P., Granholm, E. and Dionisio, D. P. (2001), "Pupillary responses and processing resources on the visual backward masking task" Psychophysiology, 38: 76–83.
- Beatty J. (1977), "Pupillometric Measurement of Cognitive Workload"