Aldehyde dehydrogenase
| Aldehyde dehydrogenase (NAD+) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
.png) Monomer of human aldehyde dehydrogenase 2 (ALDH2) with a space-filling model of NAD+ in the active site.[1] | |||||||||
| Identifiers | |||||||||
| EC number | 1.2.1.3 | ||||||||
| CAS number | 9028-86-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
Aldehyde dehydrogenases (EC 1.2.1.3) are a group of enzymes that catalyse the oxidation of aldehydes.[2] Despite the name "dehydrogenase", their mode of oxidation is by addition of oxygen rather than by removal of hydrogen – that is, they convert aldehydes (R–C(=O)–H) to carboxylic acids (R–C(=O)–O–H).[3] To date, nineteen ALDH genes have been identified within the human genome. These genes participate in a wide variety of biological processes including the detoxification of exogenously and endogenously generated aldehydes.
Function
Aldehyde dehydrogenase is a polymorphic enzyme[4] responsible for the oxidation of aldehydes to carboxylic acids, which leave the liver and are metabolized by the body’s muscle and heart.[4] There are three different classes of these enzymes in mammals: class 1 (low Km, cytosolic), class 2 (low Km, mitochondrial), and class 3 (high Km, such as those expressed in tumors, stomach, and cornea). In all three classes, constitutive and inducible forms exist. ALDH1 and ALDH2 are the most important enzymes for aldehyde oxidation, and both are tetrameric enzymes composed of 54 kDa subunits. These enzymes are found in many tissues of the body but are at the highest concentration in the liver.[4]
Active site
The active site of the aldehyde dehydrogenase enzyme is largely conserved throughout the different classes of the enzyme and, although the number of amino acids present in a subunit can change, the overall function of the site changes little. The active site binds to one molecule of an aldehyde and an NAD(P)+ that functions as a cofactor. A cysteine and a glutamate will interact with the aldehyde substrate. Many other residues will interact with the NAD(P)+ to hold it in place. A magnesium may be used to help the enzyme function, although the amount it helps the enzyme can vary between different classes of aldehydes.
|
Mechanism
The overall reaction catalysed by the aldehyde dehydrogenases is:
In this NAD(P)+-dependent reaction, the aldehyde enters the active site through a channel located on the outside of the enzyme. The active site contains a Rossman fold, and interactions between the cofactor and the fold allow for the isomerization of the enzyme while keeping the active site functional.[5]
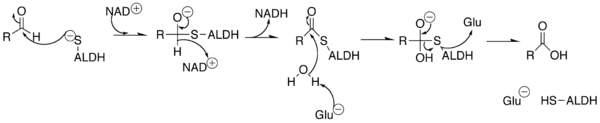 |
A sulfur from a cysteine in the active site makes a nucleophilic attack on the carbonyl carbon of the aldehyde. The hydrogen is kicked off as a hydride and attacks NAD(P)+ to make NAD(P)H. The enzyme's active site then goes through an isomorphic change whereby the NAD(P)H is moved, creating room for a water molecule to access the substrate. The water is primed by a glutamate in the active site, and the water makes a nucleophilic attack on the carbonyl carbon, kicking off the sulfur as a leaving group.
Pathology (aldehyde dehydrogenase deficiency)

ALDH2 plays a crucial role in maintaining low blood levels of acetaldehyde during alcohol oxidation. In this pathway, the intermediate structures can be toxic, and health problems arise when those intermediates cannot be cleared.[4] When high levels of acetaldehyde occur in the blood, facial flushing, lightheadedness, palpitations, nausea, and general “hangover” symptoms occur. These symptoms are indicative of a disease known as the alcohol flush reaction, also known as “Asian flush” or “Oriental flushing syndrome”.[7]
There is a mutant form of aldehyde dehydrogenase, termed ALDH2*2, wherein a lysine residue replaces a glutamate in the active site at position 487 of ALDH2.[8] Homozygous individuals with the mutant allele have almost no ALDH2 activity, and those heterozygous for the mutation have reduced activity. Thus, the mutation is partially dominant.[4] The ineffective homozygous allele works at a rate of about 8% of the normal allele, for it shows a higher km for NAD+ and has a higher maximum velocity than the wild-type allele.[4] This mutation is common in Japan, where 41% of a non-alcoholic control group were ALDH2 deficient, where only 2–5% of an alcoholic group were ALDH2-deficient. In Taiwan, the numbers are similar, with 30% of the control group showing the deficiency and 6% of alcoholics displaying it.[4] The deficiency is manifested by slow acetaldehyde removal, with low alcohol tolerance perhaps leading to a lower frequency of alcoholism.[4][7]
These symptoms are the same as those observed in people who drink while being treated by the drug disulfiram, which is why it is used to treat alcoholism. The patients show higher blood levels of acetaldehyde, and become violently ill upon consumption of even small amounts of alcohol.[4] Several drugs (e.g., metronidazole) cause a similar reaction known as "disulfiram-like reaction."
Yokoyama et al. found that decreased enzyme activity of aldehyde dehydrogenase-2, caused by the mutated ALDH2 allele, contributes to a higher chance of esophageal and oropharyngolaryngeal cancers. The metabolized acetaldehyde in the blood, which is six times higher than in individuals without the mutation, has shown to be a carcinogen in lab animals. ALDH2*2 is associated with increased odds of oropharyngolaryngeal, esophageal, gastric, colon, and lung cancer. However, they found no connection between increased levels of ALDH2*2 in the blood and an increased risk of liver cancer.[9]
Fitzmaurice et al. explored Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. "This ALDH model for PD etiology may help explain the selective vulnerability of dopaminergic neurons in PD and provide a potential mechanism through which environmental toxicants contribute to PD pathogenesis." [10]
Genes
- ALDH1A1, ALDH1A2, ALDH1A3, ALDH1B1, ALDH1L1, ALDH1L2
- ALDH2
- ALDH3A1, ALDH3A2, ALDH3B1, ALDH3B2
- ALDH4A1, ALDH5A1, ALDH6A1, ALDH7A1, ALDH8A1, ALDH9A1, ALDH16A1, ALDH18A1
See also
References
- 1 2 3 4 PDB: 1o02; Perez-Miller SJ, Hurley TD (June 2003). "Coenzyme isomerization is integral to catalysis in aldehyde dehydrogenase". Biochemistry. 42 (23): 7100–9. PMID 12795606. doi:10.1021/bi034182w.
- ↑ Marchitti, SA; Brocker, C; Stagos, D; Vasiliou, V (Jun 2008). "Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily.". Expert opinion on drug metabolism & toxicology. 4 (6): 697–720. PMC 2658643
 . PMID 18611112. doi:10.1517/17425255.4.6.697.
. PMID 18611112. doi:10.1517/17425255.4.6.697. - ↑ The oxygen comes from a water molecule. The enzyme is called a dehydrogenase because two hydrogen atoms are removed from the H2O reactant rather than from the aldehyde. By way of contrast, alcohol dehydrogenases remove hydrogens from the alcohol (R–CH2–OH) in producing an aldehyde (R–C(=O)–H).
- 1 2 3 4 5 6 7 8 9 Crabb DW, Matsumoto M, Chang D, You M (February 2004). "Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology". The Proceedings of the Nutrition Society. 63 (1): 49–63. PMID 15099407. doi:10.1079/PNS2003327.
- ↑ Liu ZJ, Sun YJ, Rose J, et al. (April 1997). "The first structure of an aldehyde dehydrogenase reveals novel interactions between NAD and the Rossmann fold". Nature Structural Biology. 4 (4): 317–26. PMID 9095201. doi:10.1038/nsb0497-317.
- ↑ Figure 11-4 in: Rod Flower; Humphrey P. Rang; Maureen M. Dale; Ritter, James M. (2007). Rang & Dale's pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-06911-5.
- 1 2 Thomasson HR, Edenberg HJ, Crabb DW, et al. (April 1991). "Alcohol and aldehyde dehydrogenase genotypes and alcoholism in Chinese men". American Journal of Human Genetics. 48 (4): 677–81. PMC 1682953
 . PMID 2014795.
. PMID 2014795. - ↑ Steinmetz CG, Xie P, Weiner H, Hurley TD (May 1997). "Structure of mitochondrial aldehyde dehydrogenase: the genetic component of ethanol aversion". Structure. 5 (5): 701–11. PMID 9195888. doi:10.1016/S0969-2126(97)00224-4.
- ↑ Yokoyama A, Muramatsu T, Ohmori T, et al. (August 1998). "Alcohol-related cancers and aldehyde dehydrogenase-2 in Japanese alcoholics". Carcinogenesis. 19 (8): 1383–7. PMID 9744533. doi:10.1093/carcin/19.8.1383.
- ↑ Fitzmaurice; et al. (January 2013). "Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease". Proc Natl Acad Sci U S A. 110 (2): 36–641. PMC 3545765
 . PMID 23267077. doi:10.1073/pnas.1220399110.
. PMID 23267077. doi:10.1073/pnas.1220399110.
External links
- Aldehyde dehydrogenase at the US National Library of Medicine Medical Subject Headings (MeSH)


