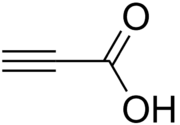
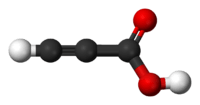
Propiolic acid
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Prop-2-ynoic acid | |
| Other names
Propiolic acid (no longer recommended[1]) Acetylene carboxylic acid Propargylic acid Acetylene mono-carboxylic acid | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.763 |
| EC Number | 207-437-8 |
| KEGG | |
| MeSH | C011537 |
| PubChem CID |
|
| |
| |
| Properties | |
| C3H2O2 | |
| Molar mass | 70.05 g/mol |
| Density | 1.1325 g/cm3 |
| Melting point | 9 °C (48 °F; 282 K) |
| Boiling point | 144 °C (291 °F; 417 K) (decomposes) |
| Hazards | |
| Safety data sheet | External MSDS |
| EU classification (DSD) (outdated) |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Propiolic acid is the organic compound with the formula HC2CO2H. It is the simplest acetylenic carboxylic acid. It is a colourless liquid that crystallises to give silky crystals. Near its boiling point, it decomposes. It is soluble in water and possesses an odor like that of acetic acid.[2]
Preparation
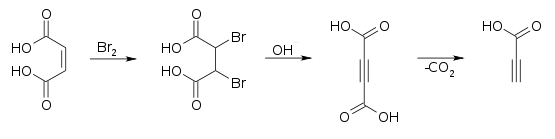
It is prepared commercially by oxidizing propargyl alcohol at a lead electrode.[3] It can also be prepared by decarboxylation of acetylenedicarboxylic acid.
Reactions and applications
Exposure to sunlight converts it into trimesic acid (benzene-1,3,5-tricarboxylic acid). It undergoes bromination to give dibromoacrylic acid. With hydrogen chloride it forms chloroacrylic acid. Its ethyl ester condenses with hydrazine to form pyrazolone.
It forms a characteristic explosive solid upon treatment to its aqueous solution with ammoniacal silver nitrate. An amorphous explosive precipitate forms with ammoniacal cuprous chloride.
Propiolates
Propiolates are esters or salts of propiolic acid. Common examples include methyl propiolate and ethyl propiolate.
See also
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 748. ISBN 978-0-85404-182-4. doi:10.1039/9781849733069-FP001.
- ↑ ed, Susan Budavari, (1990). The Merck index an encyclopedia of chemicals, drugs, and biologicals (11. ed., 2. print. ed.). Rahway, NJ: Merck. pp. 7833, 1911. ISBN 9780911910285.
- ↑ Wilhelm Riemenschneider "Carboxylic Acids, Aliphatic" Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a05_235.
-
 This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Propiolic Acid". Encyclopædia Britannica (11th ed.). Cambridge University Press.
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Propiolic Acid". Encyclopædia Britannica (11th ed.). Cambridge University Press.