Promethium(III) chloride
 Glowing powder mixture of promethium(III) chloride and zinc sulfide | |
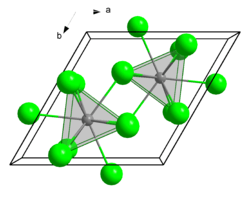 Crystal structure | |
| Names | |
|---|---|
| Other names
Promethium chloride; Promethium trichloride | |
| Identifiers | |
| ECHA InfoCard | 100.034.004 |
| Properties | |
| PmCl3 | |
| Molar mass | 253 g/mol |
| Density | 4.19 g/cm3 (calc., XRD)[1] |
| Melting point | 655 °C (1,211 °F; 928 K)[2] |
| Structure | |
| Trigonal, hP8 | |
| P63/m, No. 176[1] | |
| Related compounds | |
| Other anions |
Promethium(III) oxide |
| Other cations |
Neodymium(III) chloride, Samarium(III) chloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Promethium(III) chloride is a chemical compound of promethium and chlorine with the formula PmCl3.
Applications
Promethium(III) chloride has been used to generate long-lasting glow in signal lights and buttons. This application relied on the unstable nature of promethium, which emitted beta radiation (electrons) with a half-life of several years. The electrons were absorbed by a phosphor, generating visible glow.[3] Contrary to most other radioactive elements, promethium did not emit alpha particles that would degrade the phosphor.[4]
References
- 1 2 Weigel, F.; Scherer, V. (1967). "Die Chemie des Promethiums". Radiochimica Acta. 7. doi:10.1524/ract.1967.7.1.40.
- ↑ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.84. ISBN 1439855110.
- ↑ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.28. ISBN 1439855110.
- ↑ Lavrukhina, A. K.; Pozdnyakov, A. A. (1966). Аналитическая химия технеция, прометия, астатина и франция (Analytical Chemistry of Technetium, Promethium, Astatine, and Francium) (in Russian). Nauka. p. 118.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.