Prenylation
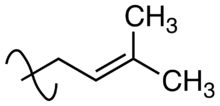
Prenylation (also known as isoprenylation or lipidation) is the addition of hydrophobic molecules to a protein or chemical compound. It is usually assumed that prenyl groups (3-methyl-but-2-en-1-yl) facilitate attachment to cell membranes, similar to lipid anchors like the GPI anchor, though direct evidence is missing. Prenyl groups have been shown to be important for protein–protein binding through specialized prenyl-binding domains.
Protein prenylation
Protein prenylation involves the transfer of either a farnesyl or a geranyl-geranyl moiety to C-terminal cysteine(s) of the target protein. There are three enzymes that carry out prenylation in the cell, farnesyl transferase, Caax protease and geranylgeranyl transferase I.[1]
Farnesylation is a type of prenylation, a post-translational modification of proteins by which an isoprenyl group is added to a cysteine residue.[2] It is an important process to mediate protein–protein interactions and protein–membrane interactions.[3]
Farnesyltransferase and geranylgeranyltransferase I
Farnesyltransferase and Geranylgeranyltransferase I are very similar proteins. They consist of two subunits, the α-subunit, which is common to both enzymes, and the β-subunit, whose sequence identity is just 25%. These enzymes recognise the CaaX box at the C-terminus of the target protein. C is the cysteine that is prenylated, a is any aliphatic amino acid, and the identity of X determines which enzyme acts on the protein. Farnesyltransferase recognizes CaaX boxes where X = M, S, Q, A, or C, whereas Geranylgeranyltransferase I recognizes CaaX boxes with X = L or E.
Rab geranylgeranyl transferase
Rab geranylgeranyltransferase, or Geranylgeranyl transferase II, transfers (usually) two geranylgeranyl groups to the cysteine(s) at the C-terminus of Rab proteins. The C-terminus of Rab proteins varies in length and sequence and is referred to as hypervariable. Thus Rab proteins do not have a consensus sequence, such as the CAAX box, which the Rab geranylgeranyl transferase can recognize. The Rab proteins usually terminate in a CC or CXC motif. Instead, Rab proteins are bound by the Rab escort protein (REP) over a more conserved region of the Rab protein and then presented to the Rab geranylgeranyltransferase. Once Rab proteins are prenylated, the lipid anchor(s) ensure that Rabs are no longer soluble. REP, therefore, plays an important role in binding and solubilising the geranylgeranyl groups and delivers the Rab protein to the relevant cell membrane.
Substrates
Both isoprenoid chains, geranylgeranyl pyrophosphate (GGpp) and farnesyl pyrophosphate are products of the HMG-CoA reductase pathway. The product of HMG CoA reductase is mevalonate. By combining precursors with 5 carbons, the pathway subsequently produces geranyl pyrophosphate (10 carbons), farnesyl pyrophosphate (15 carbons) and geranylgeranyl pyrophosphate (20 carbons). Two farnesyl pyrophosphate groups can also be combined to form squalene, the precursor for cholesterol. This means that statins, which inhibit HMG CoA reductase, inhibit the production of both cholesterol and isoprenoids.
Note that, in the HMG-CoA reductase/mevalonate pathway, the precursors already contain a pyrophosphate group, and isoprenoids are produced with a pyrophosphate group. There is no known enzyme activity that can carry out the prenylation reaction with the isoprenoid alcohol. However, enzymatic activity for isoprenoid kinases capable converting isoprenoid alcohols to isoprenoid pyrophosphates have been shown.[4] In accordance with this, farnesol and geranylgeraniol have been shown to be able to rescue effects caused by statins or nitrogenous bisphosphonates, further supporting that alcohols can be involved in prenylation, likely via phosphorylation to the corresponding isoprenoid pyrophosphate.
Proteins that undergo prenylation include Ras, which plays a central role in the development of cancer. This suggests that inhibitors of prenylation enzymes (e.g., farnesyltransferase) may influence tumor growth. In the case of the K- and N-Ras forms of Ras, when cells are treated with FTIs, these forms of Ras can undergo alternate prenylation in the form of geranylgeranylation.[5] Recent work has shown that farnesyltransferase inhibitors (FTIs) also inhibit Rab geranylgeranyltransferase and that the success of such inhibitors in clinical trials may be as much due to effects on Rab prenylation as on Ras prenylation. It should be noted that inhibitors of prenyltransferase enzymes display different specificity for the prenyltransferases, dependent upon the specific compound being utilized.
Inhibitors
FTIs can also be used to inhibit farnesylation in parasites such as trypansoma brucii and malaria. Parasites seem to be more vulnerable to inhibition of Farnesyl transferase than humans are. In some cases, this may be because they lack Geranylgeranyltransferase I. Thus, it may be possible for the development of antiparastic drugs to 'piggyback' on the development of FTIs for cancer research.
In addition, FTIs have shown some promise in treating a mouse model of progeria, and in May 2007 a phase II clinical trial using the FTI Lonafarnib was started for children with progeria.[6]
In signal transduction via G protein, palmitoylation of the α subunit, prenylation of the γ subunit, and myristoylation is involved in tethering the G protein to the inner surface of the plasma membrane so that the G protein can interact with its receptor.[7]
Small molecules prenylation
Small molecules can also undergo prenylation, such as in the case of prenylflavonoids. Recently it was described prenylation of a vitamin B2 derivative (flavin mononucleotide).[8]
Longevity and cardiac effects
A 2012 study found that statin treatment increases lifespan and improves cardiac health in Drosophila by decreasing specific protein prenylation. The study concluded, "These data are the most direct evidence to date that decreased protein prenylation can increase cardiac health and lifespan in any metazoan species, and may explain the pleiotropic (non-cholesterol related) health effects of statins."[9]
A 2012 clinical trial explored the approach of inhibiting protein prenylation with some degree of success in the treatment of Hutchinson–Gilford progeria syndrome, a multisystem disorder which causes failure to thrive and accelerated atherosclerosis leading to early death.[10][11]
See also
- Myristoylation
- Palmitoylation
- Choroideremia, a genetic disease caused by the loss of REP1, REP2 almost conmpensates, but cannot rescue the slow onset of blindness
References
- ↑ P. J. Casey & M. C. Seabra (1996). "Protein Prenyltransferases". Journal of Biological Chemistry. 271 (10): 5289–5292. PMID 8621375. doi:10.1074/jbc.271.10.5289.
- ↑ Maltese WA (December 1990). "Posttranslational modification of proteins by isoprenoids in mammalian cells". FASEB J. 4 (15): 3319–28.
- ↑ G. Novelli & M. R. D'Apice (2012). "Protein farnesylation and disease". Journal of Inherited Metabolic Disease. 35 (5): 917–926. doi:10.1007/s10545-011-9445-y.
- ↑ Bentinger, M.; Grünler, J.; Peterson, E.; Swiezewska, E.; Dallner, G. (1998). "Phosphorylation of farnesol in rat liver microsomes: properties of farnesol kinase and farnesyl phosphate kinase.". Archives of Biochemistry and Biophysics. 353 (2): 191–198. PMID 9606952. doi:10.1006/abbi.1998.0611.
- ↑ Whyte, D.; Kirschmeier, P.; Hockenberry, T.; Nunez-Oliva, I.; James, L.; Catino, J.; Bishop, W.; Pai, J. (1997). "K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors". The Journal of Biological Chemistry. 272 (22): 14459–14464. PMID 9162087. doi:10.1074/jbc.272.22.14459.
- ↑ "Phase II trial of Lonafarnib (a farnesyltransferase inhibitor) for progeria".
- ↑ Wall, MA; Coleman, DE; Lee, E; Iñiguez-Lluhi, JA; Posner, BA; Gilman, AG; Sprang, SR (15 December 1995). "The structure of the G protein heterotrimer Gi alpha 1 beta 1 gamma 2.". Cell. 83 (6): 1047–58. PMID 8521505. doi:10.1016/0092-8674(95)90220-1.
- ↑ Clarke, CF; Allan, CM. "Biochemistry: Unexpected role for vitamin B2". Nature. 522: 427–428. PMID 26083748. doi:10.1038/nature14536.
- ↑ Spindler SR, Li R, Dhahbi JM, Yamakawa A, Mote P, Bodmer R, Ocorr K, Williams RT, Wang Y, Ablao KP (2012). "Statin treatment increases lifespan and improves cardiac health in Drosophila by decreasing specific protein prenylation". PLoS ONE. 7 (6): e39581. PMC 3380867
 . PMID 22737247. doi:10.1371/journal.pone.0039581.
. PMID 22737247. doi:10.1371/journal.pone.0039581. - ↑ Gordon LB, Kleinman ME, Miller DT, Neuberg DS, Giobbie-Hurder A, Gerhard-Herman M, Smoot LB, Gordon CM, Cleveland R, Snyder BD, Fligor B, Bishop WR, Statkevich P, Regen A, Sonis A, Riley S, Ploski C, Correia A, Quinn N, Ullrich NJ, Nazarian A, Liang MG, Huh SY, Schwartzman A, Kieran MW (October 2012). "Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson–Gilford progeria syndrome". Proc Natl Acad Sci USA. 109 (41): 16666–71. PMC 3478615
 . PMID 23012407. doi:10.1073/pnas.1202529109.
. PMID 23012407. doi:10.1073/pnas.1202529109. - ↑ Young SG, Yang SH, Davies BS, Jung HJ, Fong LG (2013). "Targeting Protein Prenylation in Progeria". Sci Transl Med. 5 (171): 171ps3. PMC 3725554
 . PMID 23390246. doi:10.1126/scitranslmed.3005229.
. PMID 23390246. doi:10.1126/scitranslmed.3005229.
- ^ Maurer-Stroh, Sebastian; Eisenhaber, Frank (2005). "Refinement and prediction of protein prenylation motifs". Genome Biology. 6: R55.
- Magee A, Seabra M (2003). "Are prenyl groups on proteins sticky fingers or greasy handles?". Biochem J. 376 (Pt 2): e3–4. PMC 1223795
 . PMID 14627432. doi:10.1042/BJ20031531.
. PMID 14627432. doi:10.1042/BJ20031531. - Taylor J, Reid T, Terry K, Casey P, Beese L (2003). "Structure of mammalian protein geranylgeranyltransferase type-I". EMBO J. 22 (22): 5963–74. PMC 275430
 . PMID 14609943. doi:10.1093/emboj/cdg571.
. PMID 14609943. doi:10.1093/emboj/cdg571.
External links
- PrePS – Prenylation Prediction Suite
- Prenylation at the US National Library of Medicine Medical Subject Headings (MeSH)