Propazepine
 | |
| Names | |
|---|---|
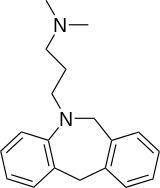
| IUPAC name
3-(6,11-dihydrobenzo[c] [1]benzazepin-5-yl)-N,N-dimethylpropan-1-amine | |
| Other names
Prazepine, Proazepine | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| PubChem CID |
|
| |
| Properties | |
| C19H24N2 | |
| Molar mass | 280.42 g·mol−1 |
| Density | 1.041 g/cm3[1] |
| Boiling point | 419.8° C[1] |
| Hazards | |
| Flash point | 188.7° C[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Propazepine is a tricyclic antidepressant (TCA).[2] Propazepine is confused with (which has the central ring nitrogen in a different location) Imipramine[2][3] Prazepine is the International nonproprietary name of this compound.[4] Unfortunately, Prazepine is also reported to be one of the many International nonproprietary names of Imipramine.[5] Propazepine can be synthesized chemically.[6][7] Propazepine appears to never have actually been used as a tricyclic antidepressant outside of initial medical tests; therefore, there is little information about it.
References
- 1 2 3 "Prazepine 73-07-4". chemnet.com.
- 1 2 Benešová, O.; Bohdanecký, Z.; Votava, Z. (1962). "Electrophysiological comparison of the action of imipramine and propazepine". Psychopharmacologia. 3 (6): 423–431. PMID 13967414. doi:10.1007/BF00411159.
- ↑ This page (pre-move) redirected from 4 April 2011 to 22 October 2015
- ↑ "Propazepine – C19H24N2". Pubchem.
- ↑ "Imipramine – C19H24N2". ChemSpider.
- ↑ Werner, L. H.; Ricca, S.; Mohacsi, E.; Rossi, A.; Arya, V. P. (1965). "Derivatives of Morphanthridine". Journal of Medicinal Chemistry. 8: 74–80. PMID 14287270. doi:10.1021/jm00325a016.
- ↑ Jílek, J. O.; Pomykáček, J.; Svátek, E.; Seidlová, V.; Rajšner, M.; Pelz, K.; Hoch, B.; Protiva, M. (1965). "Neurotrope und psychotrope substanzen II. Morphanthridin und derivate. Neue synthese des propazepins" [Neurotropic and psychotropic substances II. Morphanthridin and derivatives. New Synthesis of propazepins]. Collection of Czechoslovak Chemical Communications (in Czech). 30 (2): 445–462. doi:10.1135/cccc19650445.
Further reading
- Vojtechovsky, M; Safratova, V; Soukupova, B (1970). "The influence of propazepine on learning and memory in healthy volunteers and in patients with slight chronic brain syndrome". Activitas nervosa superior (in Czech). 12 (3): 251–2. ISSN 0001-7604. PMID 5457443.
- Rysánek, K; Vítek, V; Svehla, C (1965). "Serotonin level in human and animal thrombocytes after imipramine and propazepine administration". Activitas nervosa superior (in Czech). 7 (3): 261–2. ISSN 0001-7604. PMID 5882104.
- Souskova, M; Votava, Z; Roth, Z (1964). "Effect of Iproniazid, Propazepine, Reserpine and Their Combinations on the Orientation Reaction and Conditioned Defense Reaction in Rats". Activitas nervosa superior (in Czech). 6: 258–67. ISSN 0001-7604. PMID 14179381.
- Vinarova, M; Nahunek, K; Vencovsky, E; Bast Ecky, J; Hadlik, J (1962). "Blind comparison of clinical effects of Imipramine and Propazepine". Activitas nervosa superior (in Czech). 4: 227–8. ISSN 0001-7604. PMID 13926058.
- Souskova, M; Votava, Z (1962). "Comparative studies on the effect of Imipramine and Propazepine by a conditioned reflex method in rats". Activitas nervosa superior (in Czech). 4: 218–9. ISSN 0001-7604. PMID 13915453.
- Benesova, O; Bohdanecky, Z; Votava, Z (1962). "Comparative studies on the effect of Imipramine and Propazepine by electrophysiological methods on animals with implanted electrodes". Activitas nervosa superior (in Czech). 4: 215–6. ISSN 0001-7604. PMID 13867184.
- Metysova, J; Votava, Z (1961). "Comparison of pharmacological properties of propazepine and imipramine". Activitas nervosa superior (in Czech). 3: 227. ISSN 0001-7604. PMID 14473038.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.