Monopotassium phosphate
 | |
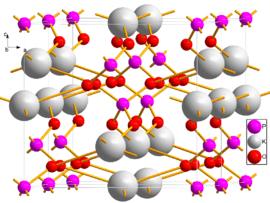 Two unit cells of MKP viewed close to the b axis | |
| Names | |
|---|---|
| IUPAC names
Potassium dihydrogenphosphate Potassium dihydrogen(tetraoxidophosphate)(1−) | |
| Systematic IUPAC name
Potassium dihydroxidodioxidophosphate(1−) | |
| Other names
Potassium phosphate monobasic; Phosphoric acid, monopotassium salt; potassium biphosphate | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.012 |
| EC Number | 231-913-4 |
| PubChem CID |
|
| RTECS number | TC6615500 |
| UNII | |
| |
| |
| Properties | |
| KH2PO4 | |
| Molar mass | 136.086 g/mol |
| Appearance | White powder deliquescent |
| Odor | odorless
pH-?? |
| Density | 2.338 g/cm3 |
| Melting point | 252.6 °C (486.7 °F; 525.8 K) |
| Boiling point | 400 °C (752 °F; 673 K) (decomposes) |
| 22.6 g/100 mL (20 °C) 83.5 g/100 mL (90 °C) | |
| Solubility | slightly soluble in ethanol |
| Acidity (pKa) | 6.86[1] |
| Basicity (pKb) | 11.9 |
| Refractive index (nD) |
1.4864 |
| Structure | |
| tetragonal[2] | |
| I42d | |
| a = 0.744 nm, b = 0.744 nm, c = 0.697 nm | |
| Hazards | |
| Safety data sheet | External MSDS |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
3200 mg/kg (rat, oral) |
| Related compounds | |
| Other cations |
Monosodium phosphate Monoammonium phosphate |
| Related compounds |
Dipotassium phosphate Tripotassium phosphate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Monopotassium phosphate, MKP, (also potassium dihydrogenphosphate, KDP, or monobasic potassium phosphate), KH2PO4, is a soluble salt of potassium and the dihydrogen phosphate ion which is used as a fertilizer, a food additive and a fungicide. It is a source of phosphorus and potassium. It is also a buffering agent. When used in fertilizer mixtures with urea and ammonium phosphates, it minimizes escape of ammonia by keeping the pH at a relatively low level.
Single crystals are paraelectric at room temperature. At temperatures below −150 °C (−238 °F) they become ferroelectric.
Structure
Monopotassium phosphate can exist in several polymorphs. At room temperature it forms paraelectric crystals with tetragonal symmetry. Upon cooling to −150 °C it transforms to a ferroelectric phase of orthorhombic symmetry, and the transition temperature shifts up to −50 °C to when hydrogen is replaced by deuterium.[3] Heating to 190 °C changes its structure to monoclinic.[4] When heated further, MKP decomposes, by loss of water, to potassium metaphosphate, KPO3, at 400 °C (752 °F).
| Symmetry | Space group |
No | Pearson symbol |
a (nm) | b (nm) | c (nm) | Z | density, g/cm3 |
T (°C) |
|---|---|---|---|---|---|---|---|---|---|
| Orthorhombic[3] | Fdd2 | 43 | oF48 | 1.0467 | 1.0533 | 0.6926 | 8 | 2.37 | < −150 |
| Tetragonal[2] | I42d | 122 | tI24 | 0.744 | 0.744 | 0.697 | 4 | 2.34 | −150 to 190 |
| Monoclinic[4] | P21/c | 14 | mP48 | 0.733 | 1.449 | 0.747 | 8 | 190 to 400 |
Manufacturing
Monopotassium phosphate is produced by the action of phosphoric acid on potassium carbonate.
Applications
Fertilizer-grade MKP powder contains the equivalent of 52% P2O5 and 34% K2O, and is labeled NPK 0-52-34. MKP powder is often used as a nutrient source in the greenhouse trade and in hydroponics.
As a crystal, MKP is noted for its non-linear optical properties. Used in optical modulators and for non-linear optics such as second-harmonic generation (SHG).
Also to be noted is KD*P, potassium dideuterium phosphate, with slightly different properties. Highly deuterated KDP is used in nonlinear frequency conversion of laser light instead of protonated (regular) KDP due to the fact that the replacement of protons with deuterons in the crystal shifts the third overtone of the strong OH molecular stretch to longer wavelengths, moving it mostly out of the range of the fundamental line at ~1064 nm of neodymium-based lasers. Regular KDP has absorbances at this wavelength of approximately 4.7–6.3%/cm of thickness while highly deuterated KDP has absorbances of typically less than 0.8%/cm.
Gallery
References
- ↑ Mathews, Christopher K., K. E. Van Holde, Ean R. Appling, and Spencer J. Anthony-Cahill. Biochemistry. Redwood City, CA: Benjamin/Cummings Pub., 1990. Print.
- 1 2 Ono, Yasuhiro; Hikita, Tomoyuki; Ikeda, Takuro (1987). "Phase Transitions in Mixed Crystal System K1−x(NH4)xH2PO4". Journal of the Physics Society Japan. 56 (2): 577. doi:10.1143/JPSJ.56.577.
- 1 2 Fukami, T. (1990). "Refinement of the Crystal Structure of KH2PO4 in the Ferroelectric Phase". Physica status solidi (a). 117 (2): K93. doi:10.1002/pssa.2211170234.
- 1 2 Itoh, Kazuyuki; Matsubayashi, Tetsuo; Nakamura, Eiji; Motegi, Hiroshi (1975). "X-Ray Study of High-Temperature Phase Transitions in KH2PO4". Journal of the Physical Society of Japan. 39 (3): 843. doi:10.1143/JPSJ.39.843.
External links
| Wikimedia Commons has media related to Monopotassium phosphate. |
- International Chemical Safety Card 1608
- EPA: Potassium dihydrogen phosphate Fact Sheet
- Potassium Phosphate – a Hydroculture Salt


