Pore-forming toxin
Pore-forming proteins (PFTs, also known as pore-forming toxins) are protein exotoxins, usually produced by bacteria, such as C. septicum and S. aureus. They are frequently cytotoxic (i.e., they kill cells), as they create unregulated pores in the membrane of targeted cells.
Types
PFTs can be divided into the following subcategories:
- Alpha-pore-forming toxins
- e.g., Cytolysin A of E. coli.
- Beta-pore-forming toxins
- e.g., α-hemolysin (Fig 1), PVL – Panton-Valentine leukocidin.
Above are the two main distinctions of PFTs. They differ in the suspected mode of membrane integration, either by alpha-helical or beta-sheet elements.[1]
Other categories:
- Binary toxins
- e.g., Anthrax toxin
- Cholesterol-dependent cytolysins (CDCs)
- e.g., Pneumolysin
- Small pore-forming toxins
- e.g., Gramicidin A
Beta-pore-forming toxins
| Leukocidin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Leukocidin | ||||||||
| Pfam | PF07968 | ||||||||
| InterPro | IPR001340 | ||||||||
| TCDB | 1.C.3 | ||||||||
| OPM superfamily | 35 | ||||||||
| OPM protein | 7ahl | ||||||||
| |||||||||
β-PFTs are so-named because of their structural characteristics: they are composed mostly of β-strand-based domains. Whilst they frequently have divergent sequences, many are classified by Pfam as Leukocidins. X-ray crystallographic structures have revealed some commonalities: α-hemolysin[2] and Panton-Valentine leukocidin S[3] are structurally related, as are aerolysin[4] and Clostridial Epsilon-toxin.[5]
Mode of action
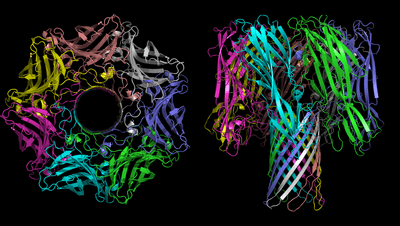
β-PFTs are dimorphic proteins that exist as soluble monomers and then assemble to form multimeric assemblies that constitute the pore. Figure 1 shows the pore-form of α-Hemolysin, the first crystal structure of a β-PFT in its pore-form. 7 α-Hemolysin monomers come together to create the mushroom-shaped pore. The 'cap' of the mushroom sits on the surface of the cell, and the 'stalk' of the mushroom penetrates the cell membrane, rendering it permeable (see later). The 'stalk' is composed of a 14-strand β-barrel, with two strands donated from each monomer.
A structure of the Vibrio cholerae cytolysin[6] in the pore form is also heptameric; however, Staphylococcus aureus gamma-hemolysin[7] reveals an octomeric pore, consequently with a 16-strand 'stalk'.
The Panton-Valentine leucocidin S structure[8] shows a highly related structure, but in its soluble monomeric state. This shows that the strands involved in forming the 'stalk' are in a very different conformation – shown in Fig 2.
Assembly
The transition between soluble monomer and membrane-associated protomer to oligomer is not a trivial one: It is believed that β-PFTs, follow as similar assembly pathway as the CDCs (see Cholesterol-dependent cytolysins later), in that they must first assemble on the cell-surface (in a receptor-mediated fashion in some cases) in a pre-pore state. Following this, the large-scale conformational change occurs in which the membrane spanning section is formed and inserted into the membrane. The portion entering the membrane, referred to as the head, is usually apolar and hydrophobic, this produces an energetically favorable insertion of the pore-forming toxin.[1]
Specificity
Some β-PFTs such as clostridial ε-toxin and Clostridium perfringens enterotoxin (CPE) bind to the cell membrane via specific receptors – possibly certain claudins for CPE,[9] possibly GPI anchors or other sugars for ε-toxin – these receptors help raise the local concentration of the toxins, allowing oligomerisation and pore formation.
The cyto-lethal effects of the pore
When the pore is formed, the tight regulation of what can and cannot enter/leave a cell is disrupted. Ions and small molecules, such as amino acids and nucleotides within the cell, flow out, and water from the surrounding tissue enters. The loss of important small molecules to the cell can disrupt protein synthesis and other crucial cellular reactions. The loss of ions, especially calcium, can cause cell signaling pathways to be spuriously activated or deactivated. The uncontrolled entry of water into a cell can cause the cell to swell up uncontrollably: this causes a process called blebbing, wherein large parts of the cell membrane are distorted and give way under the mounting internal pressure. In the end, this can cause the cell to burst.
Binary toxins
Binary toxins,[10] such as anthrax lethal and edema toxins, C. perfringens iota toxin and C. difficile cyto-lethal toxins consist of two components (hence binary):
The B component facilitates the entry of the enzymatic 'payload' into the target cell, by forming homooligomeric pores, as shown above for βPFTs. The A component then enters the cytosol and inhibits normal cell functions by one of the following means:
Mono-ADP-ribosylation of G-actin
ADP-ribosylation is a common enzymatic methods used by various bacterial toxins from various species. These toxins (including C. perfringens iota toxin and C. botulinum C2 toxin) attach a ribosyl-ADP moiety to surface arginine residue 177 of G-actin. This prevents G-actin assembling to form F-actin, and, thus, the cytoskeleton breaks down, resulting in cell death.
Proteolysis of mitogen-activated protein kinase kinases (MAPKK)
The A component of anthrax toxin lethal toxin is zinc-metalloprotease, which shows specificity for a conserved family of mitogen-activated protein kinases. The loss of these proteins results in a breakdown of cell signaling, which, in turn, renders the cell insensitive to outside stimuli – therefore no immune response is triggered.
Increasing intracellular levels of cAMP
Anthrax toxin edema toxin triggers a calcium ion influx into the target cell. This subsequently elevates intracellular cAMP levels. This can profoundly alter any sort of immune response, by inhibiting leucocyte proliferation, phagocytosis, and proinflammatory cytokine release.
Cholesterol-dependent cytolysins
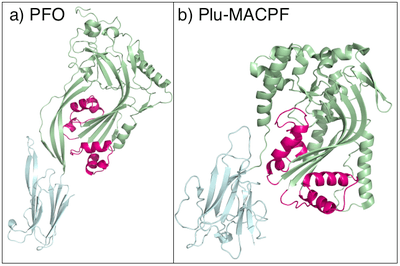
CDCs, such as pneumolysin, from S. pneumoniae, form pores as large as 260Å (26 nm), containing between 30 and 44 monomer units.[11] Electron microscopy studies of pneumolysin show that it assembles into large multimeric peripheral membrane complexes before undergoing a conformational change in which a group of α-helices in each monomer change into extended, amphipathic β-hairpins that span the membrane, in a manner reminiscent of α-haemolysin, albeit on a much larger scale (Fig 3). CDCs are homologous to the MACPF family of pore-forming toxins, and it is suggested that both families utilise a common mechanism (Fig 4).[12][13] Eukaryote MACPF proteins function in immune defence and are found in proteins such as perforin and complement C9.[14]
Biological function
Bacteria invest much time and energy in making these toxins: CPE can account for up to 15% of the dry mass of C. perfringens at the time of sporulation. The purpose of toxins is thought to be one of the following:
- Defense against phagocytosis, e.g., by a macrophage.[16]
- Inside a host, provoking a response which is beneficial for the proliferation of the bacteria, for example in cholera.[16]
- Food: After the target cell has ruptured and released its contents, the bacteria can scavenge the remains for nutrients.
- Environment: The mammalian immune response helps create the anaerobic environment that anaerobic bacteria require.
See also
References
- 1 2 Mueller, Marcus; Ulla Grauschopf; Timm Maier; Rudi Glockshuber; Nenad Ban (4 June 2009). "The structure of a cytolytic alpha-helical toxin pore reveals its assembly mechanism". Nature. 459 (7247): 726–730. PMID 19421192. doi:10.1038/nature08026.
- ↑ Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE (December 1996). "Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore". Science. 274 (5294): 1859–66. PMID 8943190. doi:10.1126/science.274.5294.1859.
- ↑ Guillet V, Roblin P, Werner S, et al. (September 2004). "Crystal structure of leucotoxin S component: new insight into the Staphylococcal β-barrel pore-forming toxins". J. Biol. Chem. 279 (39): 41028–37. PMID 15262988. doi:10.1074/jbc.M406904200.
- ↑ Parker MW, Buckley JT, Postma JP, et al. (January 1994). "Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states". Nature. 367 (6460): 292–5. PMID 7510043. doi:10.1038/367292a0.
- ↑ Cole AR, Gibert M, Popoff M, Moss DS, Titball RW, Basak AK (August 2004). "Clostridium perfringens ε-toxin shows structural similarity to the pore-forming toxin aerolysin". Nat. Struct. Mol. Biol. 11 (8): 797–8. PMID 15258571. doi:10.1038/nsmb804.
- ↑ PDB 3o44De, S.; Olson, R. (2011). "Crystal structure of the Vibrio cholerae cytolysin heptamer reveals common features among disparate pore-forming toxins". Proceedings of the National Academy of Sciences. 108 (18): 7385–7390. PMC 3088620
 . PMID 21502531. doi:10.1073/pnas.1017442108.
. PMID 21502531. doi:10.1073/pnas.1017442108. - ↑ PDB 3b07Yamashita, K.; Kawai, Y.; Tanaka, Y.; Hirano, N.; Kaneko, J.; Tomita, N.; Ohta, M.; Kamio, Y.; Yao, M.; Tanaka, I. (2011). "Crystal structure of the octameric pore of staphylococcal hemolysin reveals the barrel pore formation mechanism by two components". Proceedings of the National Academy of Sciences. 108 (42): 17314–17319. PMC 3198349
 . PMID 21969538. doi:10.1073/pnas.1110402108.
. PMID 21969538. doi:10.1073/pnas.1110402108. - ↑ PDB 1T5R Guillet, V.; Roblin, P.; Werner, S.; Coraiola, M.; Menestrina, G.; Monteil, H.; Prévost, G.; Mourey, L. (2004). "Crystal Structure of Leucotoxin S Component: NEW INSIGHT INTO THE STAPHYLOCOCCAL -BARREL PORE-FORMING TOXINS". Journal of Biological Chemistry. 279 (39): 41028–41037. PMID 15262988. doi:10.1074/jbc.M406904200.
- ↑ Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S (July 2000). "Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein". FEBS Lett. 476 (3): 258–61. PMID 10913624. doi:10.1016/S0014-5793(00)01744-0.
- ↑ Barth H, Aktories K, Popoff MR, Stiles BG (September 2004). "Binary bacterial toxins: biochemistry, biology, and applications of common Clostridium and Bacillus proteins". Microbiol. Mol. Biol. Rev. 68 (3): 373–402, table of contents. PMC 515256
 . PMID 15353562. doi:10.1128/MMBR.68.3.373-402.2004.
. PMID 15353562. doi:10.1128/MMBR.68.3.373-402.2004. - ↑ Tilley SJ, Orlova EV, Gilbert RJ, Andrew PW, Saibil HR (April 2005). "Structural basis of pore formation by the bacterial toxin pneumolysin". Cell. 121 (2): 247–56. PMID 15851031. doi:10.1016/j.cell.2005.02.033.
- 1 2 Carlos J. Rosado; Ashley M. Buckle; Ruby H. P. Law; Rebecca E. Butcher; Wan-Ting Kan; Catherina H. Bird; Kheng Ung; Kylie A. Browne; Katherine Baran; Tanya A. Bashtannyk-Puhalovich; Noel G. Faux; Wilson Wong; Corrine J. Porter; Robert N. Pike; Andrew M. Ellisdon; Mary C. Pearce; Stephen P. Bottomley; Jonas Emsley; A. Ian Smith; Jamie Rossjohn; Elizabeth L. Hartland; Ilia Voskoboinik; Joseph A. Trapani; Phillip I. Bird; Michelle A. Dunstone & James C. Whisstock (2007). "A Common Fold Mediates Vertebrate Defense and Bacterial Attack". Science. 317 (5844): 1548–51. PMID 17717151. doi:10.1126/science.1144706.
- ↑
- ↑ Tschopp J, Masson D, Stanley KK (1986). "Structural/functional similarity between proteins involved in complement- and cytotoxic T-lymphocyte-mediated cytolysis". Nature. 322 (6082): 831–4. PMID 2427956. doi:10.1038/322831a0.
- ↑ Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW (1997). "Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form". Cell. 89 (5): 685–92. PMID 9182756. doi:10.1016/S0092-8674(00)80251-2.
- 1 2 Bruce Alberts; Alexander Johnson; Julian Lewis; Martin Raff; Keith Roberts; Peter Walter (March 2002). "Molecular Biology of the Cell" (hardcover; weight 7.6 pounds) (4th ed.). Routledge. ISBN 0-8153-3218-1.
Further reading
- F. Gisou van der Goot, Pore-forming toxins, Springer, 2001, ISBN 3-540-41386-3
- A deadly toxin with a romantic name: Panton-Valentine Leukocidin complex. PDBe Quips
External links
| Wikimedia Commons has media related to Pore forming cytotoxic proteins. |
- Pore Forming Cytotoxic Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)
_and_soluble-form_PVL_(pale_green-green)_toxins_-_PDB_7AHL_and_1T5R.png)