Granulocyte
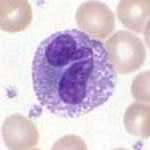
Granulocytes are a category of white blood cells characterized by the presence of granules in their cytoplasm.[1] They are also called polymorphonuclear leukocytes (PMN, PML, or PMNL) because of the varying shapes of the nucleus, which is usually lobed into three segments. This distinguishes them from the mononuclear agranulocytes. In common parlance, the term polymorphonuclear leukocyte often refers specifically to "neutrophil granulocytes",[2] the most abundant of the granulocytes; the other types (eosinophils, basophils, and mast cells) have lower numbers. Granulocytes are produced via granulopoiesis in the bone marrow.
Types
There are three principal types of granulocytes, distinguished by their appearance under Wright's stain:
Their names are derived from their staining characteristics; for example, the most abundant granulocyte is the neutrophil granulocyte, which has neutrally staining cytoplasmic granules.
Neutrophils

Neutrophils are normally found in the bloodstream and are the most abundant type of phagocyte, constituting 50% to 60% of the total circulating white blood cells.[3] One litre of human blood contains about five billion (5x109) neutrophils,[4] which are about 12–15 micrometers in diameter.[5] Once neutrophils have received the appropriate signals, it takes them about thirty minutes to leave the blood and reach the site of an infection.[6] Neutrophils do not return to the blood; they turn into pus cells and die.[6] Mature neutrophils are smaller than monocytes, and have a segmented nucleus with several sections(two to five segments); each section is connected by chromatin filaments. Neutrophils do not normally exit the bone marrow until maturity, but during an infection neutrophil precursors called myelocytes and promyelocytes are released.[7]
Neutrophils have three strategies for directly attacking micro-organisms: phagocytosis (ingestion), release of soluble anti-microbials (including granule proteins), and generation of neutrophil extracellular traps (NETs).[8] Neutrophils are professional phagocytes:[9] they are ferocious eaters and rapidly engulf invaders coated with antibodies and complement, and damaged cells or cellular debris. The intracellular granules of the human neutrophil have long been recognized for their protein-destroying and bactericidal properties.[10] Neutrophils can secrete products that stimulate monocytes and macrophages; these secretions increase phagocytosis and the formation of reactive oxygen compounds involved in intracellular killing.[11]
Neutrophils have two types of granules; primary (azurophilic) granules (found in young cells) and secondary (specific) granules (which are found in more mature cells). Primary granules contain cationic proteins and defensens that are used to kill bacteria, proteolytic enzymes and cathepsin G to break down (bacterial) proteins, lysozyme to break down bacterial cell walls, and myeloperoxidase (used to generate toxic bacteria-killing substances).[12] In addition, secretions from the primary granules of neutrophils stimulate the phagocytosis of IgG antibody-coated bacteria.[13] The secondary granules contain compounds that are involved in the formation of toxic oxygen compounds, lysozyme, and lactoferrin (used to take essential iron from bacteria).[12] Neutrophil extracellular traps (NETs) comprise a web of fibers composed of chromatin and serine proteases that trap and kill microbes extracellularly. Trapping of bacteria is a particularly important role for NETs in sepsis, where NET are formed within blood vessels.[14]
Eosinophils
Eosinophils also have kidney-shaped lobed nuclei (two to four lobes). The number of granules in an eosinophil can vary because they have a tendency to degranulate while in the blood stream.[15] Eosinophils play a crucial part in the killing of parasites (e.g., enteric nematodes) because their granules contain a unique, toxic basic protein and cationic protein (e.g., cathepsin[12]);[16] receptors that bind to IgE are used to help with this task.[17] These cells also have a limited ability to participate in phagocytosis,[18] they are professional antigen-presenting cells, they regulate other immune cell functions (e.g., CD4+ T cell, dendritic cell, B cell, mast cell, neutrophil, and basophil functions),[19] they are involved in the destruction of tumor cells,[15] and they promote the repair of damaged tissue.[20] A polypeptide called interleukin-5 interacts with eosinophils and causes them to grow and differentiate; this polypeptide is produced by basophils.[16]
Basophils
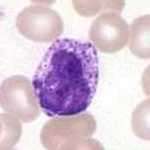
Basophils are one of the least abundant cells in bone marrow and blood (occurring at less than two percent of all cells). Like neutrophils and eosinophils, they have lobed nuclei; however, they have only two lobes, and the chromatin filaments that connect them are not very visible. Basophils have receptors that can bind to IgE, IgG, complement, and histamine. The cytoplasm of basophils contains a varied amount of granules; these granules are usually numerous enough to partially conceal the nucleus. Granule contents of basophils are abundant with histamine, heparin, chondroitin sulfate, peroxidase, platelet-activating factor, and other substances.
When an infection occurs, mature basophils will be released from the bone marrow and travel to the site of infection.[21] When basophils are injured, they will release histamine, which contributes to the inflammatory response that helps fight invading organisms. Histamine causes dilation and increased permeability of capillaries close to the basophil. Injured basophils and other leukocytes will release another substance called prostaglandins that contributes to an increased blood flow to the site of infection. Both of these mechanisms allow blood-clotting elements to be delivered to the infected area (this begins the recovery process and blocks the travel of microbes to other parts of the body). Increased permeability of the inflamed tissue also allows for more phagocyte migration to the site of infection so that they can consume microbes.[18]
Mast cells
Granulopoiesis: the genesis of granulocytes
Granulocytes are derived from stem cells residing in the bone marrow. The differentiation of these stem cells from pluripotent hematopoietic stem cell into granulocytes is termed granulopoiesis. Multiple intermediate cell types exist in this differentiation process, including myeloblasts and promyelocytes.
Toxic materials produced or released
Examples of toxic materials produced or released by degranulation by granulocytes on the ingestion of microorganism includes:
- Antimicrobial agents (Defensins and Eosinophil cationic protein)
- Enzymes
- Acid hydrolases: further digest bacteria
- Lysozyme: dissolves cell walls of some gram-positive bacteria
- Low pH vesicles (3.5-4.0)
- Toxic nitrogen oxides (nitric oxide)
- Toxic oxygen-derived products (e.g., superoxide, hydrogen peroxide, hydroxy radicals, singlet oxygen, hypohalite)
Pathology
Granulocytopenia is an abnormally low concentration of granulocytes in the blood. This condition reduces the body's resistance to many infections. Closely related terms include agranulocytosis (etymologically, "no granulocytes at all"; clinically, granulocyte levels less than 5% of normal) and neutropenia (deficiency of neutrophil granulocytes). Granulocytes live only one to two days in circulation (four days in spleen or other tissue), so transfusion of granulocytes as a therapeutic strategy would confer a very short-lasting benefit. In addition, there are many complications associated with such a procedure.
There is usually a granulocyte chemotactic defect in individuals suffering from insulin-dependent diabetes mellitus.
Additional images
 Blood cell lineage
Blood cell lineage_diagram.png) Hematopoiesis
Hematopoiesis
See also
References
- ↑ WebMD (2009). "granulocyte". Webster's New World Medical Dictionary (3rd ed.). Houghton Mifflin Harcourt. p. 181. ISBN 978-0-544-18897-6.
- ↑ WebMD (2009). "leukocyte, polymorphonuclear". Webster's New World Medical Dictionary (3rd ed.). Houghton Mifflin Harcourt. p. 244. ISBN 978-0-544-18897-6.
- ↑ Stvrtinová, Viera; Ján Jakubovský and Ivan Hulín (1995). "Neutrophils, central cells in acute inflammation". Inflammation and Fever from Pathophysiology: Principles of Disease. Computing Centre, Slovak Academy of Sciences: Academic Electronic Press. ISBN 80-967366-1-2. Retrieved March 28, 2009.
- ↑ Hoffbrand p. 331
- ↑ Abbas, Chapter 12, 5th Edition
- 1 2 Sompayrac p. 18
- ↑ Linderkamp, Otwin; Ruef, Peter; Brenner, Birgit; Gulbins, Erich; Lang, Florian (1998). "Passive Deformability of Mature, Immature, and Active Neutrophils in Healthy and Septicemic Neonates". Pediatric Research. 44 (6): 946–50. PMID 9853933. doi:10.1203/00006450-199812000-00021.
- ↑ Hickey, Michael J.; Kubes, Paul (2009). "Intravascular immunity: The host–pathogen encounter in blood vessels". Nature Reviews Immunology. 9 (5): 364–75. PMID 19390567. doi:10.1038/nri2532.
- ↑ Robinson p. 187 and Ernst pp. 7–10
- ↑ Paoletti p. 62
- ↑ Soehnlein, O.; Kenne, E.; Rotzius, P.; Eriksson, E. E.; Lindbom, L. (2007). "Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages". Clinical & Experimental Immunology. 151 (1): 139–45. PMC 2276935
 . PMID 17991288. doi:10.1111/j.1365-2249.2007.03532.x.
. PMID 17991288. doi:10.1111/j.1365-2249.2007.03532.x. - 1 2 3 Mayer, Gene (2006). "Immunology — Chapter One: Innate (non-specific) Immunity". Microbiology and Immunology On-Line Textbook. USC School of Medicine. Retrieved November 12, 2008.
- ↑ Soehnlein, Oliver; Kai-Larsen, Ylva; Frithiof, Robert; Sorensen, Ole E.; Kenne, Ellinor; Scharffetter-Kochanek, Karin; Eriksson, Einar E.; Herwald, Heiko; Agerberth, Birgitta; Lindbom, Lennart (2008). "Neutrophil primary granule proteins HBP and HNP1–3 boost bacterial phagocytosis by human and murine macrophages". Journal of Clinical Investigation. 118 (10): 3491–502. PMC 2532980
 . PMID 18787642. doi:10.1172/JCI35740.
. PMID 18787642. doi:10.1172/JCI35740. - ↑ Clark, Stephen R; Ma, Adrienne C; Tavener, Samantha A; McDonald, Braedon; Goodarzi, Zahra; Kelly, Margaret M; Patel, Kamala D; Chakrabarti, Subhadeep; McAvoy, Erin; Sinclair, Gary D; Keys, Elizabeth M; Allen-Vercoe, Emma; Devinney, Rebekah; Doig, Christopher J; Green, Francis H Y; Kubes, Paul (2007). "Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood". Nature Medicine. 13 (4): 463–69. PMID 17384648. doi:10.1038/nm1565.
- 1 2 Hess, Charles E. "Segmented Eosinophil". University of Virginia Health System. Retrieved 2009-04-10.
- 1 2 Baron, Samuel (editor) (1996). Medical Microbiology (4th edition). EditionThe University of Texas Medical Branch at Galveston. ISBN 0-9631172-1-1.
- ↑ Gleich, Gerald J.; Adolphson, Cheryl R. (1986). "The Eosinophilic Leukocyte: Structure and Function". Advances in Immunology Volume 39. Advances in Immunology. 39. pp. 177–253. ISBN 978-0-12-022439-5. doi:10.1016/S0065-2776(08)60351-X.
- 1 2 Campbell p. 903
- ↑ Akuthota, P.; Wang, H. B.; Spencer, L. A.; Weller, P. F. (2008). "Immunoregulatory roles of eosinophils: A new look at a familiar cell". Clinical & Experimental Allergy. 38 (8): 1254–63. PMC 2735457
 . PMID 18727793. doi:10.1111/j.1365-2222.2008.03037.x.
. PMID 18727793. doi:10.1111/j.1365-2222.2008.03037.x. - ↑ Kariyawasam, Harsha; Robinson, Douglas (2006). "The Eosinophil: The Cell and Its Weapons, the Cytokines, Its Locations". Seminars in Respiratory and Critical Care Medicine. 27 (2): 117–27. PMID 16612762. doi:10.1055/s-2006-939514.
- ↑ Hess, Charles E. "Mature Basophil". University of Virginia Health System. Retrieved 2009-04-10.
Bibliography
- Campbell, Neil A., Reece Jane B., Biology (6th edition), Pearson Education, Inc., 2002 ISBN 0-8053-6624-5.
- Delves, P.J., Martin, S. J., Burton, D. R. and Roit I.M. Roitt's Essential Immunology (11th edition), Blackwell Publishing, 2006, ISBN 978-1-4051-3603-7.
- Ernst J. D. and Stendahl O., (editors), Phagocytosis of Bacteria and Bacterial Pathogenicity, Cambridge University Press, 2006, ISBN 0-521-84569-6. Website.
- Hoffbrand, A.V., Pettit, J.E. and Moss, P.A.H., Essential Haematology (4th edition), Blackwell Science, 2005, ISBN 0-632-05153-1.
- Paoletti R., Notario A. and Ricevuti G., (editors), Phagocytes: Biology, Physiology, Pathology, and Pharmacotherapeutics, The New York Academy of Sciences, 1997, ISBN 1-57331-102-2.
- Robinson J.P. and Babcock G. F., (editors), Phagocyte Function —A guide for research and clinical evaluation, Wiley–Liss, 1998, ISBN 0-471-12364-1.
- Sompayrac, L. How the Immune System Works (3rd edition), Blackwell Publishing, 2008, ISBN 978-1-4051-6221-0.
External links
 Media related to Granulozyt at Wikimedia Commons
Media related to Granulozyt at Wikimedia Commons- Granulocytes at the US National Library of Medicine Medical Subject Headings (MeSH)