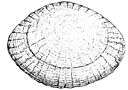
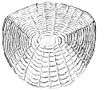
Fish scale

The skin of most fishes are covered with scales, which, in many cases, are animal reflectors or produce animal coloration. Scales vary enormously in size, shape, structure, and extent, ranging from strong and rigid armour plates in fishes such as shrimpfishes and boxfishes, to microscopic or absent in fishes such as eels and anglerfishes. The morphology of a scale can be used to identify the species of fish it came from.
Cartilaginous fishes (sharks and rays) are covered with placoid scales. Most bony fishes are covered with the cycloid scales of salmon and carp, or the ctenoid scales of perch, or the ganoid scales of sturgeons and gars. Some species are covered instead by scutes, and others have no outer covering on the skin.
Fish scales are part of the fish's integumentary system, and are produced from the mesoderm layer of the dermis, which distinguishes them from reptile scales.[1] The same genes involved in tooth and hair development in mammals are also involved in scale development. The placoid scales of cartilaginous fishes are also called dermal denticles and are structurally homologous with vertebrate teeth. It has been suggested that the scales of bony fishes are similar in structure to teeth, but they probably originate from different tissue.[2] Most fish are also covered in a protective layer of mucus (slime).
Placoid scales


Placoid scales are found in the cartilaginous fishes: sharks, rays, and chimaeras. They are also called dermal denticles. Placoid scales are structurally homologous with vertebrate teeth ("denticle" translates to "small tooth"), having a central pulp cavity supplied with blood vessels, surrounded by a conical layer of dentine, all of which sits on top of a rectangular basal plate that rests on the dermis. The outermost layer is composed of vitrodentine, a largely inorganic enamel-like substance. Placoid scales cannot grow in size, but rather more scales are added as the fish increases in size.
Similar scales can also be found under the head of the denticle herring. The amount of scale coverage is much less in rays and chimaeras.
The skin of sharks is entirely covered by placoid scales. The scales are supported by spines, which feel rough when stroked in a backward direction, but when flattened by the forward movement of water, create tiny vortices that reduce hydrodynamic drag, making swimming both more efficient as well as quieter compared to that of bony fishes. The rough, sandpaper-like texture of shark and ray skin, coupled with its toughness, has led it to be valued as a source of rawhide leather, called shagreen. One of the many historical applications of shark shagreen was in making hand-grips for swords.
Unlike bony fish, sharks have a complicated dermal corset made of flexible collagenous fibers arranged as a helical network surrounding their body. The corset works as an outer skeleton, providing attachment for their swimming muscles and thus saving energy.[3] Their dermal teeth give them hydrodynamic advantages, as the scales reduce the turbulence of swimming.[4]
Leptoid scales
Leptoid scales are found on higher-order bony fish, the teleosts (the more derived clade of ray-finned fishes). As the fish grow, scales are added in concentric layers. The scales are arranged so as to overlap in a head-to-tail configuration, like roof tiles, allowing a smoother flow of water over the body and thereby reducing drag.[5] Leptoid scales come in two forms: cycloid and ctenoid.
Cycloid scales
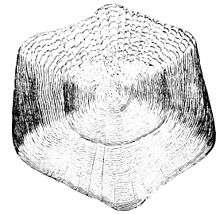

Cycloid (circular) scales have a smooth texture and are uniform, with a smooth outer edge or margin. They are most common on fish with soft fin rays, such as salmon and carp.
| Cycloid (circular) scales are usually found on carp-like or salmon-like fishes |
|
Ctenoid scales
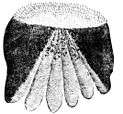


Ctenoid (toothed) scales are like cycloid scales, with small teeth along their outer edges. They are usually found on fishes with spiny fin rays, such as the perch-like fishes. The scales have a rough texture with a toothed outer or posterior edge featuring tiny teeth called ctenii. These scales contain almost no bone, being composed of a surface layer containing hydroxyapatite and calcium carbonate and a deeper layer composed mostly of collagen. The enamel of the other scale types is reduced to superficial ridges and ctenii.
| Ctenoid (toothed) scales are usually found on perch-like fishes |
|
Ctenoid scales, similar to other epidermal structures, originate from placodes and distinctive cellular differentiation makes them exclusive from other structures that arise from the integument.[6] Development starts near the caudal fin, along the lateral line of the fish.[7] The development process begins with an accumulation of fibroblasts between the epidermis and dermis.[6] Collagen fibrils begin to organize themselves in the dermal layer, which leads to the initiation of mineralization.[6] The circumference of the scales grows first, followed by thickness when overlapping layers mineralize together.[6]
Ctenoid scales can be further subdivided into three types:
- Crenate scales, where the margin of the scale bears indentations and projections.
- Spinoid scales, where the scale bears spines that are continuous with the scale itself.
- True ctenoid scales, where the spines on the scale are distinct structures.
Both cycloid and ctenoid scales are overlapping, making them more flexible than cosmoid and ganoid scales. Unlike ganoid scales, they grow in size through additions to the margin. The scales of some species exhibit bands of uneven seasonal growth called annuli (singular annulus). These bands can be used to age the fish. Most ray-finned fishes have ctenoid scales. Some species of flatfishes have ctenoid scales on the eyed side and cycloid scales on the blind side, while other species have ctenoid scales in males and cycloid scales in females.
Ganoid scales
_(3149758934).jpg)
Ganoid scales are found in the sturgeons, paddlefishes, gars, bowfin, and bichirs. They are derived from cosmoid scales, with a layer of dentine in the place of cosmine, and a layer of inorganic bone salt called ganoine in place of vitrodentine. Most are diamond-shaped and connected by peg-and-socket joints. They are usually thick and have a minimal amount of overlap as compared to other scales.[8] In sturgeons, the scales are greatly enlarged into armour plates along the sides and back, while in the bowfin the scales are greatly reduced in thickness to resemble cycloid scales (see above).
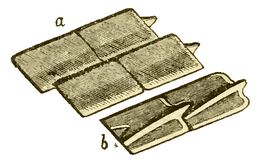 |
Ganoid scales of the Carboniferous fish, Amblypterus striatus. (a) shows the outer surface of four of the scales, and (b) shows the inner surface of two of the scales. Each of the rhomboidal ganoid scales of Amblypterus has a ridge on the inner surface which is produced at one end into a projecting peg which fits into a notch in the next scale, similar to the manner in which tiles are pegged together on the roof of a house. |
Elasmoid scales

Elasmoid scales are thin, imbricated scales composed of a layer of dense, lamellar bone called isopedine, above which is a layer of tubercles usually composed of bone, as in Eusthenopteron. The layer of dentine that was present in the first sarcopterygians is usually reduced, as in the extant coelacanth, or entirely absent, as in extant lungfish and in the Devonian Eusthenopteron.[9] Elasmoid scales have appeared several times over the course of fish evolution. They are present in some lobe-finned fishes: coelacanths, all extant and some extinct lungfishes, some tetrapodomorphs like Eusthenopteron, amiids, and teleosts, whose cycloid and ctenoid scales represent the least mineralized elasmoid scales.
Cosmoid scales
Cosmoid scales are found in several ancient lobe-finned fishes, including some of the earliest lungfishes, and were probably derived from a fusion of placoid scales. They are composed of a layer of dense, lamellar bone called isopedine, above which is a layer of spongy bone supplied with blood vessels. The bone layers are covered by a complex dentine layer called cosmine and a superficial outer coating of vitrodentine. Cosmoid scales increase in size through the growth of the lamellar bone layer.
Scutes
A scute is another, less common, type of scale. Scute comes from Latin for shield, and can take the form of:
- an external shield-like bony plate, or
- a modified, thickened scale that often is keeled or spiny, or
- a projecting, modified (rough and strongly ridged) scale, usually associated with the lateral line, or on the caudal peduncle forming caudal keels, or along the ventral profile.
Some fish, such as pineconefish, are completely or partially covered in scutes. River herrings and threadfins have an abdominal row of scutes, which are scales with raised, sharp points that are used for protection. Some jacks have a row of scutes following the lateral line on either side.
Thelodont scales

The bony scales of thelodonts, the most abundant form of fossil fish, are well understood. The scales were formed and shed throughout the organisms' lifetimes, and quickly separated after their death.[10]
Bone, a tissue that is both resistant to mechanical damage and relatively prone to fossilization, often preserves internal detail, which allows the histology and growth of the scales to be studied in detail. The scales comprise a non-growing "crown" composed of dentine, with a sometimes-ornamented enameloid upper surface and an aspidine base.[11] Its growing base is made of cell-free bone, which sometimes developed anchorage structures to fix it in the side of the fish.[12] Beyond that, there appear to be five types of bone-growth, which may represent five natural groupings within the thelodonts—or a spectrum ranging between the end members meta- (or ortho-) dentine and mesodentine tissues.[13] Interestingly, each of the five scale morphs appears to resemble the scales of more derived groupings of fish, suggesting that thelodont groups may have been stem groups to succeeding clades of fish.[12]
However, using scale morphology alone to distinguish species has some pitfalls. Within each organism, scale shape varies hugely according to body area,[14] with intermediate forms appearing between different areas—and to make matters worse, scale morphology may not even be constant within one area. To confuse things further, scale morphologies are not unique to taxa, and may be indistinguishable on the same area of two different species.[15]
The morphology and histology of thelodonts provides the main tool for quantifying their diversity and distinguishing between species, although ultimately using such convergent traits is prone to errors. Nonetheless, a framework comprising three groups has been proposed based upon scale morphology and histology.[13]
Modifications
Different groups of fish have evolved a number of modified scales to serve various functions.
- Almost all fishes have a lateral line, a system of mechanoreceptors that detect water movements. In bony fishes, the scales along the lateral line have central pores that allow water to contact the sensory cells.
- The dorsal fin spines of dogfish sharks and chimaeras, the stinging tail spines of stingrays, and the "saw" teeth of sawfishes and sawsharks are fused and modified placoid scales.
- Porcupine fishes have scales modified into spines.
- Surgeonfishes have a sharp, blade-like spines on either side of the caudal peduncle.
- Some herrings, anchovies, and halfbeaks have deciduous scales, which are easily shed and aid in escaping predators.
- Male Percina darters have a row of enlarged caducous scales between the pelvic fins and the anus.
Many groups of bony fishes, including pipefishes and seahorses, several families of catfishes, sticklebacks, and poachers, have developed external bony plates, structurally resembling placoid scales, as protective armour. In the boxfishes, the plates are all fused together to form a rigid shell enclosing the entire body. Yet these bony plates are not modified scales, but skin that has been ossified.
 |
 |
| The size of the teeth on ctenoid scales can vary with position, as these scales from the rattail Cetonurus crassiceps show | |
| |
 |
| Eels seem scaleless, yet some species are covered with tiny smooth cycloid scales | |
 Scales of Arapaima gigas
Scales of Arapaima gigas- Scales of European bitterling from the hindflank
See also
- Identification of aging in fish
- Reptile scale
- Snake scales
- Scale (zoology)
- Urokotori – Japanese fish scaler
- Animal coloration
- Animal reflectors
- photonic crystals
References
- ↑ Sharpe, P. T. (2001). "Fish scale development: Hair today, teeth and scales yesterday?". Current Biology. 11 (18): R751–R752. PMID 11566120. doi:10.1016/S0960-9822(01)00438-9.
- ↑ The First False Teeth
- ↑ Martin, R. Aidan. "The Importance of Being Cartilaginous". ReefQuest Centre for Shark Research. Retrieved 2009-08-29.
- ↑ Martin, R. Aidan. "Skin of the Teeth". Retrieved 2007-08-28.
- ↑ Ballard, Bonnie; Cheek, Ryan (2 July 2016). Exotic Animal Medicine for the Veterinary Technician. John Wiley & Sons. ISBN 978-1-118-92421-1.
- 1 2 3 4 Kawasaki, Kenta C., "A Genetic Analysis of Cichlid Scale Morphology" (2016). Masters Theses May 2014 - current. 425. http://scholarworks.umass.edu/masters_theses_2/425
- ↑ Helfman, Gene (2009). The Diversity of Fishes Biology, Evolution, and Ecology. Wiley-Blackwell.
- ↑ Sherman, Vincent R.; Yaraghi, Nicholas A.; Kisailus, David; Meyers, Marc A. (2016-12-01). "Microstructural and geometric influences in the protective scales of Atractosteus spatula". Journal of The Royal Society Interface. 13 (125): 20160595. ISSN 1742-5689. PMID 27974575. doi:10.1098/rsif.2016.0595.
- ↑ Zylberberg, L., Meunier, F.J., Laurin, M. (2010). A microanatomical and histological study of the postcranial dermal skeleton in the Devonian sarcopterygian Eusthenopteron foordi, Acta Palaeontologica Polonica 55: 459–470.
- ↑ Turner, S.; Tarling, D. H. (1982). "Thelodont and other agnathan distributions as tests of Lower Paleozoic continental reconstructions". Palaeogeography, Palaeoclimatology, Palaeoecology. 39 (3–4): 295–311. doi:10.1016/0031-0182(82)90027-X.
- ↑ Märss, T. (2006). "Exoskeletal ultrasculpture of early vertebrates". Journal of Vertebrate Paleontology. 26 (2): 235–252. doi:10.1671/0272-4634(2006)26[235:EUOEV]2.0.CO;2.
- 1 2 Janvier, Philippe (1998). "Early vertebrates and their extant relatives". Early Vertebrates. Oxford University Press. pp. 123–127. ISBN 0-19-854047-7.
- 1 2 Turner, S. (1991). "Monophyly and interrelationships of the Thelodonti". In M. M. Chang, Y. H. Liu & G. R. Zhang. Early Vertebrates and Related Problems of Evolutionary Biology. Science Press, Beijing. pp. 87–119.
- ↑ Märss, T. (1986). "Squamation of the thelodont agnathan Phlebolepis". Journal of Vertebrate Paleontology. 6 (1): 1–11. doi:10.1080/02724634.1986.10011593.
- ↑ Botella, H.; J. I. Valenzuela-Rios; P. Carls (2006). "A New Early Devonian thelodont from Celtiberia (Spain), with a revision of Spanish thelodonts". Palaeontology. 49 (1): 141–154. doi:10.1111/j.1475-4983.2005.00534.x.
Further reading
- Helfman, G.S., B.B. Collette and D.E. Facey (1997). The Diversity of Fishes. Blackwell Science. pp. 33–36. ISBN 978-0-86542-256-8.