Pigment


A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light.
Many materials selectively absorb certain wavelengths of light. Materials that humans have chosen and developed for use as pigments usually have special properties that make them ideal for coloring other materials. A pigment must have a high tinting strength relative to the materials it colors. It must be stable in solid form at ambient temperatures.
For industrial applications, as well as in the arts, permanence and stability are desirable properties. Pigments that are not permanent are called fugitive. Fugitive pigments fade over time, or with exposure to light, while some eventually blacken.
Pigments are used for coloring paint, ink, plastic, fabric, cosmetics, food, and other materials. Most pigments used in manufacturing and the visual arts are dry colorants, usually ground into a fine powder. This powder is added to a binder (or vehicle), a relatively neutral or colorless material that suspends the pigment and gives the paint its adhesion.
A distinction is usually made between a pigment, which is insoluble in its vehicle (resulting in a suspension), and a dye, which either is itself a liquid or is soluble in its vehicle (resulting in a solution). A colorant can act as either a pigment or a dye depending on the vehicle involved. In some cases, a pigment can be manufactured from a dye by precipitating a soluble dye with a metallic salt. The resulting pigment is called a lake pigment. The term biological pigment is used for all colored substances independent of their solubility.[1]
In 2006, around 7.4 million tons of inorganic, organic and special pigments were marketed worldwide. Asia has the highest rate on a quantity basis followed by Europe and North America. By 2020, revenues will have risen to approx. US$34.2 billion.[2] The global demand on pigments was roughly US$20.5 billion in 2009, around 1.5-2% up from the previous year. It is predicted to increase in a stable growth rate in the coming years. The worldwide sales are said to increase up to US$24.5 billion in 2015, and reach US$27.5 billion in 2018.[3]
Physical basis

Pigments appear the colors they are because they selectively reflect and absorb certain wavelengths of visible light. White light is a roughly equal mixture of the entire spectrum of visible light with a wavelength in a range from about 375 or 400 nanometers to about 760 or 780 nm. When this light encounters a pigment, parts of the spectrum are absorbed by the molecules or ions of the pigment. In organic pigments such as diazo or phthalocyanine compounds the light is absorbed by the conjugated systems of double bonds in the molecule. Some of the inorganic pigments such as vermilion (mercury sulfide) or cadmium yellow (cadmium sulfide) absorb light by transferring an electron from the negative ion (S2−) to the positive ion (Hg2+ or Cd2+). Such compounds are designated as charge-transfer complexes,[4] with broad absorption bands that subtract most of the colors of the incident white light. The other wavelengths or parts of the spectrum are reflected or scattered. The new reflected light spectrum creates the appearance of a color. Pigments, unlike fluorescent or phosphorescent substances, can only subtract wavelengths from the source light, never add new ones.
The appearance of pigments is intimately connected to the color of the source light. Sunlight has a high color temperature, and a fairly uniform spectrum, and is considered a standard for white light. Artificial light sources tend to have great peaks in some parts of their spectrum, and deep valleys in others. Viewed under these conditions, pigments will appear different colors.
Color spaces used to represent colors numerically must specify their light source. Lab color measurements, unless otherwise noted, assume that the measurement was taken under a D65 light source, or "Daylight 6500 K", which is roughly the color temperature of sunlight.
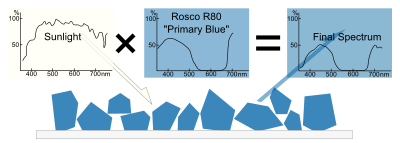
Other properties of a color, such as its saturation or lightness, may be determined by the other substances that accompany pigments. Binders and fillers added to pure pigment chemicals also have their own reflection and absorption patterns, which can affect the final spectrum. Likewise, in pigment/binder mixtures, individual rays of light may not encounter pigment molecules, and may be reflected as is. These stray rays of source light contribute to a slightly less saturated color. Pure pigment allows very little white light to escape, producing a highly saturated color. A small quantity of pigment mixed with a lot of white binder, however, will appear desaturated and pale, due to the high quantity of escaping white light.
History
Naturally occurring pigments such as ochres and iron oxides have been used as colorants since prehistoric times. Archaeologists have uncovered evidence that early humans used paint for aesthetic purposes such as body decoration. Pigments and paint grinding equipment believed to be between 350,000 and 400,000 years old have been reported in a cave at Twin Rivers, near Lusaka, Zambia.[5]
Before the Industrial Revolution, the range of color available for art and decorative uses was technically limited. Most of the pigments in use were earth and mineral pigments, or pigments of biological origin. Pigments from unusual sources such as botanical materials, animal waste, insects, and mollusks were harvested and traded over long distances. Some colors were costly or impossible to obtain, given the range of pigments that were available. Blue and purple came to be associated with royalty because of their rarity.
Biological pigments were often difficult to acquire, and the details of their production were kept secret by the manufacturers. Tyrian Purple is a pigment made from the mucus of one of several species of Murex snail. Production of Tyrian Purple for use as a fabric dye began as early as 1200 BCE by the Phoenicians, and was continued by the Greeks and Romans until 1453 CE, with the fall of Constantinople.[6] The pigment was expensive and complex to produce, and items colored with it became associated with power and wealth. Greek historian Theopompus, writing in the 4th century BCE, reported that "purple for dyes fetched its weight in silver at Colophon [in Asia Minor]."[7]
Mineral pigments were also traded over long distances. The only way to achieve a deep rich blue was by using a semi-precious stone, lapis lazuli, to produce a pigment known as ultramarine, and the best sources of lapis were remote. Flemish painter Jan van Eyck, working in the 15th century, did not ordinarily include blue in his paintings. To have one's portrait commissioned and painted with ultramarine blue was considered a great luxury. If a patron wanted blue, they were obliged to pay extra. When Van Eyck used lapis, he never blended it with other colors. Instead he applied it in pure form, almost as a decorative glaze.[8] The prohibitive price of lapis lazuli forced artists to seek less expensive replacement pigments, both mineral (azurite, smalt) and biological (indigo).

Spain's conquest of a New World empire in the 16th century introduced new pigments and colors to peoples on both sides of the Atlantic. Carmine, a dye and pigment derived from a parasitic insect found in Central and South America, attained great status and value in Europe. Produced from harvested, dried, and crushed cochineal insects, carmine could be, and still is, used in fabric dye, food dye, body paint, or in its solid lake form, almost any kind of paint or cosmetic.
According to Diana Magaloni, the Florentine Codex contains a variety of illustrations with multiple variations of the red pigments. Specifically in the case of achiotl (light red), technical analysis of the paint reveals multiple layers of the pigment although the layers of the pigment is not visible to the naked eye. Therefore, it proves that the process of applying multiple layers is more significant in comparison to the actual color itself. Furthermore, the process of layering the various hues of the same pigment on top of each other enabled the Aztec artists to create variations in the intensity of the subject matter. A bolder application of pigment draws the viewer’s eye to the subject matter which commands attention and suggests a power of the viewer. A weaker application of pigment commands less attention and has less power. This would suggest that the Aztec associated the intensity of pigments with the idea of power and life.[9]
Natives of Peru had been producing cochineal dyes for textiles since at least 700 CE,[10] but Europeans had never seen the color before. When the Spanish invaded the Aztec empire in what is now Mexico, they were quick to exploit the color for new trade opportunities. Carmine became the region's second most valuable export next to silver. Pigments produced from the cochineal insect gave the Catholic cardinals their vibrant robes and the English "Redcoats" their distinctive uniforms. The true source of the pigment, an insect, was kept secret until the 18th century, when biologists discovered the source.[11]

While carmine was popular in Europe, blue remained an exclusive color, associated with wealth and status. The 17th-century Dutch master Johannes Vermeer often made lavish use of lapis lazuli, along with carmine and Indian yellow, in his vibrant paintings.
Development of synthetic pigments
The earliest known pigments were natural minerals. Natural iron oxides give a range of colors and are found in many Paleolithic and Neolithic cave paintings. Two examples include Red Ochre, anhydrous Fe2O3, and the hydrated Yellow Ochre (Fe2O3.H2O).[12] Charcoal, or carbon black, has also been used as a black pigment since prehistoric times.[12]
Two of the first synthetic pigments were white lead (basic lead carbonate, (PbCO3)2Pb(OH)2)[13] and blue frit (Egyptian Blue). White lead is made by combining lead with vinegar (acetic acid, CH3COOH) in the presence of CO2. Blue frit is calcium copper silicate and was made from glass colored with a copper ore, such as malachite. These pigments were used as early as the second millennium BCE[14] Later premodern additions to the range of synthetic pigments included vermillion, verdigris and lead-tin-yellow.
The Industrial and Scientific Revolutions brought a huge expansion in the range of synthetic pigments, pigments that are manufactured or refined from naturally occurring materials, available both for manufacturing and artistic expression. Because of the expense of Lapis Lazuli, much effort went into finding a less costly blue pigment.
Prussian Blue was the first modern synthetic pigment, discovered by accident in 1704.[15] By the early 19th century, synthetic and metallic blue pigments had been added to the range of blues, including French ultramarine, a synthetic form of lapis lazuli, and the various forms of Cobalt and Cerulean Blue. In the early 20th century, organic chemistry added Phthalo Blue, a synthetic, organometallic pigment with overwhelming tinting power.

Discoveries in color science created new industries and drove changes in fashion and taste. The discovery in 1856 of mauveine, the first aniline dye, was a forerunner for the development of hundreds of synthetic dyes and pigments like azo and diazo compounds which are the source of a wide spectrum of colors. Mauveine was discovered by an 18-year-old chemist named William Henry Perkin, who went on to exploit his discovery in industry and become wealthy. His success attracted a generation of followers, as young scientists went into organic chemistry to pursue riches. Within a few years, chemists had synthesized a substitute for madder in the production of Alizarin Crimson. By the closing decades of the 19th century, textiles, paints, and other commodities in colors such as red, crimson, blue, and purple had become affordable.[16]
Development of chemical pigments and dyes helped bring new industrial prosperity to Germany and other countries in northern Europe, but it brought dissolution and decline elsewhere. In Spain's former New World empire, the production of cochineal colors employed thousands of low-paid workers. The Spanish monopoly on cochineal production had been worth a fortune until the early 19th century, when the Mexican War of Independence and other market changes disrupted production.[17] Organic chemistry delivered the final blow for the cochineal color industry. When chemists created inexpensive substitutes for carmine, an industry and a way of life went into steep decline.[18]
New sources for historic pigments

Before the Industrial Revolution, many pigments were known by the location where they were produced. Pigments based on minerals and clays often bore the name of the city or region where they were mined. Raw Sienna and Burnt Sienna came from Siena, Italy, while Raw Umber and Burnt Umber came from Umbria. These pigments were among the easiest to synthesize, and chemists created modern colors based on the originals that were more consistent than colors mined from the original ore bodies. But the place names remained.
Historically and culturally, many famous natural pigments have been replaced with synthetic pigments, while retaining historic names. In some cases, the original color name has shifted in meaning, as a historic name has been applied to a popular modern color. By convention, a contemporary mixture of pigments that replaces a historical pigment is indicated by calling the resulting color a hue, but manufacturers are not always careful in maintaining this distinction. The following examples illustrate the shifting nature of historic pigment names:
- Indian Yellow was once produced by collecting the urine of cattle that had been fed only mango leaves.[20] Dutch and Flemish painters of the 17th and 18th centuries favored it for its luminescent qualities, and often used it to represent sunlight. Since mango leaves are nutritionally inadequate for cattle, the practice of harvesting Indian Yellow was eventually declared to be inhumane.[20] Modern hues of Indian Yellow are made from synthetic pigments.
- Ultramarine, originally the semi-precious stone lapis lazuli, has been replaced by an inexpensive modern synthetic pigment, French Ultramarine, manufactured from aluminium silicate with sulfur impurities. At the same time, Royal Blue, another name once given to tints produced from lapis lazuli, has evolved to signify a much lighter and brighter color, and is usually mixed from Phthalo Blue and titanium dioxide, or from inexpensive synthetic blue dyes. Since synthetic ultramarine is chemically identical with lapis lazuli, the "hue" designation is not used. French Blue, yet another historic name for ultramarine, was adopted by the textile and apparel industry as a color name in the 1990s, and was applied to a shade of blue that has nothing in common with the historic pigment ultramarine.
- Vermilion, a toxic mercury compound favored for its deep red-orange color by old master painters such as Titian, has been replaced in painters' palettes by various modern pigments, including cadmium reds. Although genuine Vermilion paint can still be purchased for fine arts and art conservation applications, few manufacturers make it, because of legal liability issues. Few artists buy it, because it has been superseded by modern pigments that are both less expensive and less toxic, as well as less reactive with other pigments. As a result, genuine Vermilion is almost unavailable. Modern vermilion colors are properly designated as Vermilion Hue to distinguish them from genuine Vermilion.
Manufacturing and industrial standards
Before the development of synthetic pigments, and the refinement of techniques for extracting mineral pigments, batches of color were often inconsistent. With the development of a modern color industry, manufacturers and professionals have cooperated to create international standards for identifying, producing, measuring, and testing colors.
First published in 1905, the Munsell color system became the foundation for a series of color models, providing objective methods for the measurement of color. The Munsell system describes a color in three dimensions, hue, value (lightness), and chroma (color purity), where chroma is the difference from gray at a given hue and value.
By the middle years of the 20th century, standardized methods for pigment chemistry were available, part of an international movement to create such standards in industry. The International Organization for Standardization (ISO) develops technical standards for the manufacture of pigments and dyes. ISO standards define various industrial and chemical properties, and how to test for them. The principal ISO standards that relate to all pigments are as follows:
- ISO-787 General methods of test for pigments and extenders.
- ISO-8780 Methods of dispersion for assessment of dispersion characteristics.
Other ISO standards pertain to particular classes or categories of pigments, based on their chemical composition, such as ultramarine pigments, titanium dioxide, iron oxide pigments, and so forth.
Many manufacturers of paints, inks, textiles, plastics, and colors have voluntarily adopted the Colour Index International (CII) as a standard for identifying the pigments that they use in manufacturing particular colors. First published in 1925, and now published jointly on the web by the Society of Dyers and Colourists (United Kingdom) and the American Association of Textile Chemists and Colorists (USA), this index is recognized internationally as the authoritative reference on colorants. It encompasses more than 27,000 products under more than 13,000 generic color index names.
In the CII schema, each pigment has a generic index number that identifies it chemically, regardless of proprietary and historic names. For example, Phthalocyanine Blue BN has been known by a variety of generic and proprietary names since its discovery in the 1930s. In much of Europe, phthalocyanine blue is better known as Helio Blue, or by a proprietary name such as Winsor Blue. An American paint manufacturer, Grumbacher, registered an alternate spelling (Thanos Blue) as a trademark. Colour Index International resolves all these conflicting historic, generic, and proprietary names so that manufacturers and consumers can identify the pigment (or dye) used in a particular color product. In the CII, all phthalocyanine blue pigments are designated by a generic color index number as either PB15 or PB16, short for pigment blue 15 and pigment blue 16; these two numbers reflect slight variations in molecular structure that produce a slightly more greenish or reddish blue.
Scientific and technical issues
Selection of a pigment for a particular application is determined by cost, and by the physical properties and attributes of the pigment itself. For example, a pigment that is used to color glass must have very high heat stability in order to survive the manufacturing process; but, suspended in the glass vehicle, its resistance to alkali or acidic materials is not an issue. In artistic paint, heat stability is less important, while lightfastness and toxicity are greater concerns.
The following are some of the attributes of pigments that determine their suitability for particular manufacturing processes and applications:
- Lightfastness and sensitivity for damage from ultra violet light
- Heat stability
- Toxicity
- Tinting strength
- Staining
- Dispersion
- Opacity or transparency
- Resistance to alkalis and acids
- Reactions and interactions between pigments
Swatches
Swatches are used to communicate colors accurately. For different media like printing, computers, plastics, and textiles, different type of swatches are used. Generally, the medium which offers the broadest gamut of color shades is widely used across different media.
Printed swatches
There are many reference standards providing printed swatches of color shades. PANTONE, RAL, Munsell etc. are widely used standards of color communication across different media like printing, plastics, and textiles.
Plastic swatches
Companies manufacturing color masterbatches and pigments for plastics offer plastic swatches in injection molded color chips. These color chips are supplied to the designer or customer to choose and select the color for their specific plastic products.
Plastic swatches are available in various special effects like pearl, metallic, fluorescent, sparkle, mosaic etc. However, these effects are difficult to replicate on other media like print and computer display. wherein they have created plastic swatches on website by 3D modelling to including various special effects.
Computer swatches
Pure pigments reflect light in a very specific way that cannot be precisely duplicated by the discrete light emitters in a computer display. However, by making careful measurements of pigments, close approximations can be made. The Munsell Color System provides a good conceptual explanation of what is missing. Munsell devised a system that provides an objective measure of color in three dimensions: hue, value (or lightness), and chroma. Computer displays in general are unable to show the true chroma of many pigments, but the hue and lightness can be reproduced with relative accuracy. However, when the gamma of a computer display deviates from the reference value, the hue is also systematically biased.
The following approximations assume a display device at gamma 2.2, using the sRGB color space. The further a display device deviates from these standards, the less accurate these swatches will be.[21] Swatches are based on the average measurements of several lots of single-pigment watercolor paints, converted from Lab color space to sRGB color space for viewing on a computer display. Different brands and lots of the same pigment may vary in color. Furthermore, pigments have inherently complex reflectance spectra that will render their color appearance greatly different depending on the spectrum of the source illumination; a property called metamerism. Averaged measurements of pigment samples will only yield approximations of their true appearance under a specific source of illumination. Computer display systems use a technique called chromatic adaptation transforms[22] to emulate the correlated color temperature of illumination sources, and cannot perfectly reproduce the intricate spectral combinations originally seen. In many cases, the perceived color of a pigment falls outside of the gamut of computer displays and a method called gamut mapping is used to approximate the true appearance. Gamut mapping trades off any one of lightness, hue, or saturation accuracy to render the color on screen, depending on the priority chosen in the conversion's ICC rendering intent.
|
#990024
|
PR106 - #E34234
Vermilion (genuine)
|
#FFB02E
|
|---|
|
PB29 - #003BAF
Ultramarine blue
|
PB27 - #0B3E66
|
|---|
Biological pigments
In biology, a pigment is any colored material of plant or animal cells. Many biological structures, such as skin, eyes, fur, and hair contain pigments (such as melanin). Animal skin coloration often comes about through specialized cells called chromatophores, which animals such as the octopus and chameleon can control to vary the animal's color. Many conditions affect the levels or nature of pigments in plant, animal, some protista, or fungus cells. For instance, the disorder called albinism affects the level of melanin production in animals.
Pigmentation in organisms serves many biological purposes, including camouflage, mimicry, aposematism (warning), sexual selection and other forms of signalling, photosynthesis (in plants), as well as basic physical purposes such as protection from sunburn.
Pigment color differs from structural color in that pigment color is the same for all viewing angles, whereas structural color is the result of selective reflection or iridescence, usually because of multilayer structures. For example, butterfly wings typically contain structural color, although many butterflies have cells that contain pigment as well.
Pigments by elemental composition
Metal-based pigments
- Cadmium pigments: cadmium yellow, cadmium red, cadmium green, cadmium orange, cadmium sulfoselenide
- Chromium pigments: chrome yellow and chrome green|viridian
- Cobalt pigments: cobalt violet, cobalt blue, cerulean blue, aureolin (cobalt yellow)
- Copper pigments: Azurite, Han purple, Han blue, Egyptian blue, Malachite, Paris green, Phthalocyanine Blue BN, Phthalocyanine Green G, verdigris
- Iron oxide pigments: sanguine, caput mortuum, oxide red, red ochre, Venetian red, Prussian blue
- Lead pigments: lead white, cremnitz white, Naples yellow, red lead, lead-tin-yellow
- Manganese pigments: manganese violet
- Mercury pigments: vermilion
- Titanium pigments: titanium yellow, titanium beige, titanium white, titanium black
- Zinc pigments: zinc white, zinc ferrite, zinc yellow
Other inorganic pigments
- Carbon pigments: carbon black (including vine blac, lamp black), ivory black (bone char)
- Clay earth pigments (iron oxides): yellow ochre, raw sienna, burnt sienna, raw umber, burnt umber.
- Ultramarine pigments: ultramarine, ultramarine green shade
Biological and organic
- Biological origins: alizarin (synthesized), alizarin crimson (synthesized), gamboge, cochineal red, rose madder, indigo, Indian yellow, Tyrian purple
- Non biological organic: quinacridone, magenta, phthalo green, phthalo blue, pigment red 170, diarylide yellow
See also
Notes
- ↑ Khan, Amina (14 October 2011). "Artifacts indicate a 100,000-year-old art studio" – via LA Times.
- ↑ Market Study Pigments, 3rd ed., Ceresana, 11/13
- ↑ "Market Report: World Pigment Market". Acmite Market Intelligence.
- ↑ Thomas B. Brill, Light:Its Interaction with Art and Antiquities, Springer 1980, p. 204
- ↑ "Earliest evidence of art found". BBC News. 2000-05-02. Retrieved 2016-05-01.
- ↑ Kassinger, Ruth G. (2003-02-06). Dyes: From Sea Snails to Synthetics. 21st century. ISBN 0-7613-2112-8.
- ↑ Theopompus, cited by Athenaeus [12.526] in c. 200 BCE; according to Gulick, Charles Barton. (1941). Athenaeus, The Deipnosophists. Cambridge: Harvard University Press.
- ↑ Michel Pastoureau (2001-10-01). Blue: The History of a Color. Princeton University Press. ISBN 0-691-09050-5.
- ↑ Magaloni Kerpel, Diana (2014). The Colors of The New World. Los Angeles, CA: The Getty Research Institute. pp. 35–40.
- ↑ Jan Wouters, Noemi Rosario-Chirinos (1992). "Dye Analysis of Pre-Columbian Peruvian Textiles with High-Performance Liquid Chromatography and Diode-Array Detection". Journal of the American Institute for Conservation. The American Institute for Conservation of Historic &. 31 (2): 237–255. JSTOR 3179495. doi:10.2307/3179495.
- ↑ Amy Butler Greenfield (2005-04-26). A Perfect Red: Empire, Espionage, and the Quest for the Color of Desire. HarperCollins. ISBN 0-06-052275-5.
- 1 2 "Pigments Through the Ages". WebExhibits.org. Retrieved 2007-10-18.
- ↑ Lead white at ColourLex
- ↑ Rossotti, Hazel (1983). Colour: Why the World Isn't Grey. Princeton, NJ: Princeton University Press. ISBN 0-691-02386-7.
- ↑ Prussian blue at ColourLex
- ↑ Simon Garfield (2000). Mauve: How One Man Invented a Color That Changed the World. Faber and Faber. ISBN 0-393-02005-3.
- ↑ Octavio Hernández. "Cochineal". Mexico Desconocido Online. Retrieved July 15, 2005.
- ↑ Jeff Behan. "The bug that changed history". Retrieved June 26, 2006.
- ↑ Johannes Vermeer, The Milkmaid, ColourLex
- 1 2 "History of Indian yellow". Pigments Through the Ages. Retrieved 13 February 2015.
- ↑ "Dictionary of Color Terms". Gamma Scientific. Retrieved 2014-06-25.
- ↑ "Chromatic Adaptation". cmp.uea.ac.uk. Retrieved 2009-04-16.
References
- Ball, Philip (2002). Bright Earth: Art and the Invention of Color. Farrar, Straus and Giroux. ISBN 0-374-11679-2.
- Doerner, Max (1984). The Materials of the Artist and Their Use in Painting: With Notes on the Techniques of the Old Masters, Revised Edition. Harcourt. ISBN 0-15-657716-X.
- Finlay, Victoria (2003). Color: A Natural History of the Palette. Random House. ISBN 0-8129-7142-6.
- Gage, John (1999). Color and Culture: Practice and Meaning from Antiquity to Abstraction. University of California Press. ISBN 0-520-22225-3.
- Meyer, Ralph (1991). The Artist's Handbook of Materials and Techniques, Fifth Edition. Viking. ISBN 0-670-83701-6.
- L. Feller, L. Ed., Artists' Pigments. A Handbook of Their History and Characteristics, Vol. 1, Cambridge University Press, London 1986.
- Roy A. Ed., Artists' Pigments. A Handbook of Their History and Characteristics, Vol. 2, Oxford University Press 1993.
- Fitzhugh, E.W. Ed. Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 3: Oxford University Press 1997.
- Berrie, B. Ed., Artists' Pigments. A Handbook of Their History and Characteristics, Vol. 4, Archetype books, 2007.
External links
| Wikimedia Commons has media related to Pigments. |
- Pigments through the ages
- ColourLex Pigment Lexicon
- Meyrs, G.D., An Artists Paint and Pigment Reference with Color Index Names, Color Index Numbers and Chemical Composition
- Earliest evidence of art found
- Sarah Lowengard,The Creation of Color in Eighteenth-century Europe, Columbia University Press, 2006
- Phillip Ball, (audio) On Chemistry and Colour in Art
- An Overview On A Pigment Phthalo Green Organic Pigments
- Alchemy's Rainbow: Pigment Science and the Art of Conservation on YouTube, Chemical Heritage Foundation
- Poisons and Pigments: A Talk with Art Historian Elisabeth Berry-Drago on YouTube, Chemical Heritage Foundation