Phytoremediation
Phytoremediation /ˌfaɪtəʊrɪˌmiːdɪˈeɪʃən/ (from Ancient Greek φυτό (phyto), meaning 'plant', and Latin remedium, meaning 'restoring balance') refers to the technologies that use living plants to clean up soil, air, and water contaminated with hazardous chemicals.[1]
Phytoremediation is a cost-effective plant-based approach of remediation that takes advantage of the ability of plants to concentrate elements and compounds from the environment and to metabolize various molecules in their tissues. It refers to the natural ability of certain plants called hyperaccumulators to bioaccumulate, degrade, or render harmless contaminants in soils, water, or air. Toxic heavy metals and organic pollutants are the major targets for phytoremediation. Knowledge of the physiological and molecular mechanisms of phytoremediation began to emerge in recent years together with biological and engineering strategies designed to optimize and improve phytoremediation. In addition, several field trials confirmed the feasibility of using plants for environmental cleanup.[2]
Application
Phytoremediation may be applied wherever the soil or static water environment has become polluted or is suffering ongoing chronic pollution. Examples where phytoremediation has been used successfully include the restoration of abandoned metal mine workings, and sites where polychlorinated biphenyls have been dumped during manufacture and mitigation of ongoing coal mine discharges reducing the impact of contaminants in soils, water, or air. Contaminants such as metals, pesticides, solvents, explosives,[3] and crude oil and its derivatives, have been mitigated in phytoremediation projects worldwide. Many plants such as mustard plants, alpine pennycress, hemp, and pigweed have proven to be successful at hyperaccumulating contaminants at toxic waste sites.
Over the past 20 years, this technology has become increasingly popular and has been employed at sites with soils contaminated with lead, uranium, and arsenic. While it has the advantage that environmental concerns may be treated in situ; one major disadvantage of phytoremediation is that it requires a long-term commitment, as the process is dependent on a plant's ability to grow and thrive in an environment that is not ideal for normal plant growth.
Advantages and limitations
- Advantages:
- the cost of the phytoremediation is lower than that of traditional processes both in situ and ex situ
- the plants can be easily monitored
- the possibility of the recovery and re-use of valuable metals (by companies specializing in "phyto mining")
- it is potentially the least harmful method because it uses naturally occurring organisms and preserves the environment in a more natural state.
- Limitations:
- phytoremediation is limited to the surface area and depth occupied by the roots.
- slow growth and low biomass require a long-term commitment
- with plant-based systems of remediation, it is not possible to completely prevent the leaching of contaminants into the groundwater (without the complete removal of the contaminated ground, which in itself does not resolve the problem of contamination)
- the survival of the plants is affected by the toxicity of the contaminated land and the general condition of the soil.
- bio-accumulation of contaminants, especially metals, into the plants which then pass into the food chain, from primary level consumers upwards or requires the safe disposal of the affected plant material.
Various processes
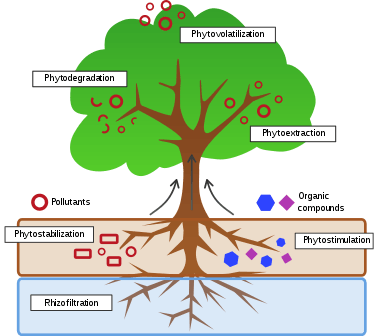
A range of processes mediated by plants or algae are useful in treating environmental problems:
- Phytosequestration – Phytochemical complexation in the root zone, reduce the fraction of the contaminant that is bioavailable. Transport protein inhibition on the root membrane-preventing contaminants from entering the plant. Vacuolar storage in the root cells: contaminants can be sequestered into the vacuoles of root cells.
- Phytoextraction – uptake and concentration of substances from the environment into the plant biomass.
- Phytostabilization – reducing the mobility of substances in the environment, for example, by limiting the leaching of substances from the soil.
- Phytotransformation – chemical modification of environmental substances as a direct result of plant metabolism, often resulting in their inactivation, degradation (phytodegradation), or immobilization (phytostabilization).
- Phytostimulation – enhancement of soil microbial activity for the degradation of contaminants, typically by organisms that associate with roots. This process is also known as rhizosphere degradation. Phytostimulation can also involve aquatic plants supporting active populations of microbial degraders, as in the stimulation of atrazine degradation by hornwort.[4]
- Phytovolatilization – removal of substances from soil or water with release into the air, sometimes as a result of phytotransformation to more volatile and/or less polluting substances.
- Rhizofiltration – filtering water through a mass of roots to remove toxic substances or excess nutrients. The pollutants remain absorbed in or adsorbed to the roots.
- Biological hydraulic containment – Some plants, like poplars, draws water upwards through the soil into the roots and out through the plant decreases the movement of soluble contaminants downwards, deeper into the site and into the groundwater.[5]
Phytoextraction
Phytoextraction (or phytoaccumulation) uses plants or algae to remove contaminants from soils, sediments or water into harvestable plant biomass (organisms that take larger-than-normal amounts of contaminants from the soil are called hyperaccumulators). Phytoextraction has been growing rapidly in popularity worldwide for the last twenty years or so. In general, this process has been tried more often for extracting heavy metals than for organics. At the time of disposal, contaminants are typically concentrated in the much smaller volume of the plant matter than in the initially contaminated soil or sediment. 'Mining with plants', or phytomining, is also being experimented with:
The plants absorb contaminants through the root system and store them in the root biomass and/or transport them up into the stems and/or leaves. A living plant may continue to absorb contaminants until it is harvested. After harvest, a lower level of the contaminant will remain in the soil, so the growth/harvest cycle must usually be repeated through several crops to achieve a significant cleanup. After the process, the cleaned soil can support other vegetation.
Advantages
The main advantage of phytoextraction is environmental friendliness. Traditional methods that are used for cleaning up heavy metal-contaminated soil disrupt soil structure and reduce soil productivity, whereas phytoextraction can clean up the soil without causing any kind of harm to soil quality. Another benefit of phytoextraction is that it is less expensive than any other clean-up process.
Disadvantages
As this process is controlled by plants, it takes more time than anthropogenic soil clean-up methods.
Two versions
- natural hyper-accumulation, where plants naturally take up the contaminants in soil unassisted.
- induced or assisted hyper-accumulation, where a conditioning fluid containing a chelator or another agent is added to soil to increase metal solubility or mobilization so that the plants can absorb them more easily. In many cases natural hyperaccumulators are metallophyte plants that can tolerate and incorporate high levels of toxic metals.
Examples
- Arsenic, using the sunflower (Helianthus annuus),[6] or the Chinese Brake fern (Pteris vittata).[7]
- Cadmium, using willow (Salix viminalis): In 1999, one research experiment performed by Maria Greger and Tommy Landberg suggested willow has a significant potential as a phytoextractor of cadmium (Cd), zinc (Zn), and copper (Cu), as willow has some specific characteristics like high transport capacity of heavy metals from root to shoot and huge amount of biomass production; can be used also for production of bio energy in the biomass energy power plant.[8]
- Cadmium and zinc, using alpine pennycress (Thlaspi caerulescens), a hyperaccumulator of these metals at levels that would be toxic to many plants. On the other hand, the presence of copper seems to impair its growth (see table for reference).
- Lead, using Indian mustard (Brassica juncea), ragweed (Ambrosia artemisiifolia), hemp dogbane (Apocynum cannabinum), or poplar trees, which sequester lead in their biomass.
- Salt-tolerant (moderately halophytic) barley and/or sugar beets are commonly used for the extraction of sodium chloride (common salt) to reclaim fields that were previously flooded by sea water.
- Caesium-137 and strontium-90 were removed from a pond using sunflowers after the Chernobyl accident.[9]
- Mercury, selenium and organic pollutants such as polychlorinated biphenyls (PCBs) have been removed from soils by transgenic plants containing genes for bacterial enzymes.[10]
Phytostabilization
Phytostabilization focuses on the long term stabilization and containment of the pollutant. For example, the plant's presence can reduce wind erosion; or the plant's roots can prevent water erosion, immobilize the pollutants by adsorption or accumulation, and provide a zone around the roots where the pollutant can precipitate and stabilize. Unlike phytoextraction, phytostabilization focuses mainly on sequestering pollutants in soil near the roots but not in plant tissues. Pollutants become less bioavailable, and livestock, wildlife, and human exposure is reduced. An example application of this sort is using a vegetative cap to stabilize and contain mine tailings.[11]
Phytotransformation
In the case of organic pollutants, such as pesticides, explosives, solvents, industrial chemicals, and other xenobiotic substances, certain plants, such as Cannas, render these substances non-toxic by their metabolism.[12] In other cases, microorganisms living in association with plant roots may metabolize these substances in soil or water. These complex and recalcitrant compounds cannot be broken down to basic molecules (water, carbon-dioxide, etc.) by plant molecules, and, hence, the term phytotransformation represents a change in chemical structure without complete breakdown of the compound. The term "Green Liver" is used to describe phytotransformation,[13] as plants behave analogously to the human liver when dealing with these xenobiotic compounds (foreign compound/pollutant).[14][15] After uptake of the xenobiotics, plant enzymes increase the polarity of the xenobiotics by adding functional groups such as hydroxyl groups (-OH).
This is known as Phase I metabolism, similar to the way that the human liver increases the polarity of drugs and foreign compounds (drug metabolism). Whereas in the human liver enzymes such as cytochrome P450s are responsible for the initial reactions, in plants enzymes such as peroxidases, phenoloxidases, esterases and nitroreductases carry out the same role.[12]
In the second stage of phytotransformation, known as Phase II metabolism, plant biomolecules such as glucose and amino acids are added to the polarized xenobiotic to further increase the polarity (known as conjugation). This is again similar to the processes occurring in the human liver where glucuronidation (addition of glucose molecules by the UGT class of enzymes, e.g. UGT1A1) and glutathione addition reactions occur on reactive centres of the xenobiotic.
Phase I and II reactions serve to increase the polarity and reduce the toxicity of the compounds, although many exceptions to the rule are seen. The increased polarity also allows for easy transport of the xenobiotic along aqueous channels.
In the final stage of phytotransformation (Phase III metabolism), a sequestration of the xenobiotic occurs within the plant. The xenobiotics polymerize in a lignin-like manner and develop a complex structure that is sequestered in the plant. This ensures that the xenobiotic is safely stored, and does not affect the functioning of the plant. However, preliminary studies have shown that these plants can be toxic to small animals (such as snails), and, hence, plants involved in phytotransformation may need to be maintained in a closed enclosure.
Hence, the plants reduce toxicity (with exceptions) and sequester the xenobiotics in phytotransformation. Trinitrotoluene phytotransformation has been extensively researched and a transformation pathway has been proposed.[16]
Role of genetics
Breeding programs and genetic engineering are powerful methods for enhancing natural phytoremediation capabilities, or for introducing new capabilities into plants. Genes for phytoremediation may originate from a micro-organism or may be transferred from one plant to another variety better adapted to the environmental conditions at the cleanup site. For example, genes encoding a nitroreductase from a bacterium were inserted into tobacco and showed faster removal of TNT and enhanced resistance to the toxic effects of TNT.[17] Researchers have also discovered a mechanism in plants that allows them to grow even when the pollution concentration in the soil is lethal for non-treated plants. Some natural, biodegradable compounds, such as exogenous polyamines, allow the plants to tolerate concentrations of pollutants 500 times higher than untreated plants, and to absorb more pollutants.
Hyperaccumulators and biotic interactions
A plant is said to be a hyperaccumulator if it can concentrate the pollutants in a minimum percentage which varies according to the pollutant involved (for example: more than 1000 mg/kg of dry weight for nickel, copper, cobalt, chromium or lead; or more than 10,000 mg/kg for zinc or manganese).[18] This capacity for accumulation is due to hypertolerance, or phytotolerance: the result of adaptative evolution from the plants to hostile environments through many generations. A number of interactions may be affected by metal hyperaccumulation, including protection, interferences with neighbour plants of different species, mutualism (including mycorrhizae, pollen and seed dispersal), commensalism, and biofilm.
Table of hyperaccumulators
- Hyperaccumulators table – 1 : Al, Ag, As, Be, Cr, Cu, Mn, Hg, Mo, Naphthalene, Pb, Pd, Pt, Se, Zn
- Hyperaccumulators table – 2 : Nickel
- Hyperaccumulators table – 3 : Radionuclides (Cd, Cs, Co, Pu, Ra, Sr, U), Hydrocarbons, Organic Solvents.
Phytoscreening
As plants are able to translocate and accumulate particular types of contaminants, plants can be used as biosensors of subsurface contamination, thereby allowing investigators to quickly delineate contaminant plumes.[19][20] Chlorinated solvents, such as trichloroethylene, have been observed in tree trunks at concentrations related to groundwater concentrations.[21] To ease field implementation of phytoscreening, standard methods have been developed to extract a section of the tree trunk for later laboratory analysis, often by using an increment borer.[22] Phytoscreening may lead to more optimized site investigations and reduce contaminated site cleanup costs.
See also
- Phytoremediation plants
- Phytotreatment
- Biodegradation
- Bioremediation
- Bioaugmentation
References
- ↑ Reichenauer TG, Germida JJ (2008). "Phytoremediation of organic contaminants in soil and groundwater". Chemsuschem. 1 (8-9): 708–17. PMID 18698569. doi:10.1002/cssc.200800125.
- ↑ Salt DE, Smith RD, Raskin I (1998). "PHYTOREMEDIATION". Annual Review of Plant Physiology and Plant Molecular Biology. 49: 643–668. PMID 15012249. doi:10.1146/annurev.arplant.49.1.643.
- ↑ Phytoremediation of soils using Ralstonia eutropha, Pseudomas tolaasi, Burkholderia fungorum reported by Sofie Thijs
- ↑ Rupassara, S. I.; Larson, R. A.; Sims, G. K. & Marley, K. A. (2002), "Degradation of Atrazine by Hornwort in Aquatic Systems", Bioremediation Journal, 6 (3): 217–224, doi:10.1080/10889860290777576.
- ↑ Evans, Gareth M.; Furlong, Judith C. (2010-01-01). Phytotechnology and Photosynthesis. John Wiley & Sons, Ltd. pp. 145–174. ISBN 9780470975152. doi:10.1002/9780470975152.ch7/summary.
- ↑ Marchiol, L.; Fellet, G.; Perosa, D.; Zerbi, G. (2007), "Removal of trace metals by Sorghum bicolor and Helianthus annuus in a site polluted by industrial wastes: A field experience", Plant Physiology and Biochemistry, 45 (5): 379–87, PMID 17507235, doi:10.1016/j.plaphy.2007.03.018
- ↑ Wang, J.; Zhao, FJ; Meharg, AA; Raab, A; Feldmann, J; McGrath, SP (2002), "Mechanisms of Arsenic Hyperaccumulation in Pteris vittata. Uptake Kinetics, Interactions with Phosphate, and Arsenic Speciation", Plant Physiology, 130 (3): 1552–61, PMC 166674
 , PMID 12428020, doi:10.1104/pp.008185
, PMID 12428020, doi:10.1104/pp.008185 - ↑ Greger, M. & Landberg, T. (1999), "Using of Willow in Phytoextraction", International Journal of Phytoremediation, 1 (2): 115–123, doi:10.1080/15226519908500010.
- ↑ Adler, Tina (July 20, 1996). "Botanical cleanup crews: using plants to tackle polluted water and soil". Science News. Retrieved 2010-09-03.
- ↑ Meagher, RB (2000), "Phytoremediation of toxic elemental and organic pollutants", Current Opinion in Plant Biology, 3 (2): 153–162, PMID 10712958, doi:10.1016/S1369-5266(99)00054-0.
- ↑ Mendez MO, Maier RM (2008), "Phytostabilization of Mine Tailings in Arid and Semiarid Environments—An Emerging Remediation Technology", Environ Health Perspect, 116 (3): 278–83, PMC 2265025
 , PMID 18335091, doi:10.1289/ehp.10608, archived from the original on 2008-10-24.
, PMID 18335091, doi:10.1289/ehp.10608, archived from the original on 2008-10-24. - 1 2 Kvesitadze, G.; et al. (2006), Biochemical Mechanisms of Detoxification in Higher Plants, Berlin, Heidelberg: Springer, ISBN 978-3-540-28996-8
- ↑ Sanderman, H. (1994), "Higher plant metabolism of xenobiotics: the "green liver" concept", Pharmacogenetics, 4: 225–241, doi:10.1097/00008571-199410000-00001.
- ↑ Burken, J.G. (2004), "2. Uptake and Metabolism of Organic Compounds: Green-Liver Model", in McCutcheon, S.C.; Schnoor, J.L., Phytoremediation: Transformation and Control of Contaminants, A Wiley-Interscience Series of Texts and Monographs, Hoboken, NJ: John Wiley, p. 59, ISBN 0-471-39435-1, doi:10.1002/047127304X.ch2
- ↑ Ramel, F., Sulmon, C., Serra, A.A., Gouesbet, G., Couée I. (2012), “Xenobiotic sensing and signalling in higher plants”, Journal of Experimental Botany, 63(11):3999-4014, doi: 10.1093/jxb/ers102, PMID 22493519
- ↑ Subramanian, Murali; Oliver, David J. & Shanks, Jacqueline V. (2006), "TNT Phytotransformation Pathway Characteristics in Arabidopsis: Role of Aromatic Hydroxylamines", Biotechnol. Prog., 22 (1): 208–216, PMID 16454512, doi:10.1021/bp050241g.
- ↑ Hannink, N.; Rosser, S. J.; French, C. E.; Basran, A.; Murray, J. A.; Nicklin, S.; Bruce, N. C. (2001), "Phytodetoxification of TNT by transgenic plants expressing a bacterial nitroreductase", Nature Biotechnology, 19 (12): 1168–72, PMID 11731787, doi:10.1038/nbt1201-1168.
- ↑ Baker, A. J. M.; Brooks, R. R. (1989), "Terrestrial higher plants which hyperaccumulate metallic elements – A review of their distribution, ecology and phytochemistry", Biorecovery, 1 (2): 81–126.
- ↑ Burken, J.; Vroblesky, D.; Balouet, J.C. (2011), "Phytoforensics, Dendrochemistry, and Phytoscreening: New Green Tools for Delineating Contaminants from Past and Present", Environmental Science & Technology, 45 (15): 6218–6226, doi:10.1021/es2005286.
- ↑ Sorek, A.; Atzmon, N.; Dahan, O.; Gerstl, Z.; Kushisin, L.; Laor, Y.; Mingelgrin, U.; Nasser, A.; Ronen, D.; Tsechansky, L.; Weisbrod, N.; Graber, E.R. (2008), ""Phytoscreening": The Use of Trees for Discovering Subsurface Contamination by VOCs", Environmental Science & Technology, 42 (2): 536–542, doi:10.1021/es072014b.
- ↑ Vroblesky, D.; Nietch, C.; Morris, J. (1998), "Chlorinated Ethenes from Groundwater in Tree Trunks", Environmental Science & Technology, 33 (3): 510–515, doi:10.1021/es980848b.
- ↑ Vroblesky, D. (2008). "User's Guide to the Collection and Analysis of Tree Cores to Assess the Distribution of Subsurface Volatile Organic Compounds".
Bibliography
- "Phytoremediation Website" — Includes reviews, conference announcements, lists of companies doing phytoremediation, and bibliographies.
- "An Overview of Phytoremediation of Lead and Mercury" June 6 2000. The Hazardous Waste Clean-Up Information Web Site.
- "Enhanced phytoextraction of arsenic from contaminated soil using sunflower" September 22 2004. U.S. Environmental Protection Agency.
- "Phytoextraction", February 2000. Brookhaven National Laboratory 2000.
- "Phytoextraction of Metals from Contaminated Soil" April 18, 2001. M.M. Lasat
- July 2002. Donald Bren School of Environment Science & Management.
- "Phytoremediation" October 1997. Department of Civil Environmental Engineering.
- "Phytoremediation" June 2001, Todd Zynda.
- "Phytoremediation of Lead in Residential Soils in Dorchester, MA" May, 2002. Amy Donovan Palmer, Boston Public Health Commission.
- "Technology Profile: Phytoextraction" 1997. Environmental Business Association.
- Vassil AD, Kapulnik Y, Raskin I, Salt DE (June 1998), "The Role of EDTA in Lead Transport and Accumulation by Indian Mustard", Plant Physiol., 117 (2): 447–53, PMC 34964
 , PMID 9625697, doi:10.1104/pp.117.2.447.
, PMID 9625697, doi:10.1104/pp.117.2.447. - Salt, D. E.; Smith, R. D.; Raskin, I. (1998). "Phytoremediation". Annual Review of Plant Physiology and Plant Molecular Biology. 49: 643–668. PMID 15012249. doi:10.1146/annurev.arplant.49.1.643.
- K. Oh, T. Li, H. Y. Cheng, Y. Xie, and S. Yonemochi., "Development of Profitable Phytoremediation of Contaminated Soils with Biofuel Crops," Journal of Environmental Protection, vol. 4, pp. 58–64, 2013.
- X. J. Wang, F. Y. Li, M. Okazaki, and M. Sugisaki, "Phytoremediation of contaminated soil", Annual Report CESS, vol. 3, pp. 114–123, 2003.
External links
| Look up phytoremediation in Wiktionary, the free dictionary. |
- Missouri Botanical Garden (host): Phytoremediation website — Review Articles, Conferences, Phytoremediation Links, Research Sponsors, Books and Journals, and Recent Research.
- International Journal of Phytoremediation — devoted to the publication of current laboratory and field research describing the use of plant systems to remediate contaminated environments.
- Using Plants To Clean Up Soils — from Agricultural Research magazine
- New Alchemy Institute — co-founded by John Todd (biologist)