Silicate minerals
| Silicate minerals | |
|---|---|
|
Copper silicate mineral chrysocolla | |
| Category | Mineral |
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of rock-forming minerals and make up approximately 90 percent of the Earth's crust.[1] They are classified based on the structure of their silicate groups, which contain different ratios of silicon and oxygen.
Nesosilicates or orthosilicates
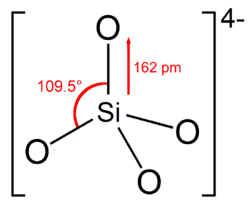

Nesosilicates (from Greek νῆσος nēsos, island), or orthosilicates, have the orthosilicate ion, which constitute isolated (insular) [SiO4]4− tetrahedra that are connected only by interstitial cations. Nickel–Strunz classification: 09.A
Examples are:
- Phenakite group
- Olivine group
- Forsterite – Mg2SiO4
- Fayalite – Fe2SiO4
- Tephroite – Mn2SiO4
- Garnet group
- Pyrope – Mg3Al2(SiO4)3
- Almandine – Fe3Al2(SiO4)3
- Spessartine – Mn3Al2(SiO4)3
- Grossular – Ca3Al2(SiO4)3
- Andradite – Ca3Fe2(SiO4)3
- Uvarovite – Ca3Cr2(SiO4)3
- Hydrogrossular – Ca3Al2Si2O8(SiO4)3−m(OH)4m
- Zircon group

- Al2SiO5 group
- Andalusite – Al2SiO5
- Kyanite – Al2SiO5
- Sillimanite – Al2SiO5
- Dumortierite – Al6.5–7BO3(SiO4)3(O,OH)3
- Topaz – Al2SiO4(F,OH)2
- Staurolite – Fe2Al9(SiO4)4(O,OH)2
- Humite group – (Mg,Fe)7(SiO4)3(F,OH)2
- Norbergite – Mg3(SiO4)(F,OH)2
- Chondrodite – Mg5(SiO4)2(F,OH)2
- Humite – Mg7(SiO4)3(F,OH)2
- Clinohumite – Mg9(SiO4)4(F,OH)2
- Datolite – CaBSiO4(OH)
- Titanite – CaTiSiO5
- Chloritoid – (Fe,Mg,Mn)2Al4Si2O10(OH)4
- Mullite (aka Porcelainite) – Al6Si2O13
Sorosilicates

Sorosilicates (from Greek σωρός sōros, heap, mound) have isolated double tetrahedra groups with (Si2O7)6− or a ratio of 2:7. Nickel–Strunz classification: 09.B
Examples are:
- Hemimorphite (calamine) – Zn4(Si2O7)(OH)2·H2O
- Lawsonite – CaAl2(Si2O7)(OH)2·H2O
- Axinite – (Ca,Fe,Mn)3Al2(BO3)(Si4O12)(OH)
- Ilvaite – CaFeII2FeIIIO(Si2O7)(OH)
- Epidote group (has both (SiO4)4− and (Si2O7)6− groups)
- Epidote – Ca2(Al,Fe)3O(SiO4)(Si2O7)(OH)
- Zoisite – Ca2Al3O(SiO4)(Si2O7)(OH)
- Tanzanite – Ca2Al3O(SiO4)(Si2O7)(OH)
- Clinozoisite – Ca2Al3O(SiO4)(Si2O7)(OH)
- Allanite – Ca(Ce,La,Y,Ca)Al2(FeII,FeIII)O(SiO4)(Si2O7)(OH)
- Dollaseite-(Ce) – CaCeMg2AlSi3O11F(OH)
- Vesuvianite (idocrase) – Ca10(Mg,Fe)2Al4(SiO4)5(Si2O7)2(OH)4
Cyclosilicates

Cyclosilicates (from Greek κύκλος kuklos, circle), or ring silicates, have linked tetrahedra with (TxO3x)2x− or a ratio of 1:3. These exist as 3-member (T3O9)6− and 6-member (T6O18)12− rings, where T stands for a tetrahedrally coordinated cation. Nickel–Strunz classification: 09.C
Examples are:
- 3-member ring
- Benitoite – BaTi(Si3O9)
- 6-member ring
- Beryl – Be3Al2(Si6O18)
- Bazzite – Be3Sc2(Si6O18)
- Sugilite – KNa2(Fe,Mn,Al)2Li3Si12O30
- Tourmaline – (Na,Ca)(Al,Li,Mg)3−(Al,Fe,Mn)6(Si6O18(BO3)3(OH)4
- Pezzottaite – Cs(Be2Li)Al2Si6O18
- Milarite – K2Ca4Al2Be4(Si24O60)H2O
- Osumilite – (K,Na)(Fe,Mg)2(Al,Fe)3(Si,Al)12O30
- Cordierite – (Mg, Fe)2Al4Si5O18
- Sekaninaite – (Fe+2, Mg)2Al4Si5O18
Note that the ring in axinite contains two B and four Si tetrahedra and is highly distorted compared to the other 6-member ring cyclosilicates.
 Cyclosilicate, [Si6O18] – 6-membered single rings, beryl (red: Si, blue: O)
Cyclosilicate, [Si6O18] – 6-membered single rings, beryl (red: Si, blue: O) Cyclosilicate, [Si3O9] – 3-membered single ring, benitoite
Cyclosilicate, [Si3O9] – 3-membered single ring, benitoite Cyclosilicate, [Si4O12] – 4-membered single ring, papagoite
Cyclosilicate, [Si4O12] – 4-membered single ring, papagoite Cyclosilicate, [Si9O27] – 9-membered ring, eudialyte
Cyclosilicate, [Si9O27] – 9-membered ring, eudialyte Cyclosilicate, [Si6O18] – 6-membered double ring, milarite
Cyclosilicate, [Si6O18] – 6-membered double ring, milarite

Inosilicates
Inosilicates (from Greek ἴς is [genitive: ἰνός inos], fibre), or chain silicates, have interlocking chains of silicate tetrahedra with either SiO3, 1:3 ratio, for single chains or Si4O11, 4:11 ratio, for double chains. Nickel–Strunz classification: 09.D
Examples are:
Single chain inosilicates
- Pyroxene group
- Enstatite – orthoferrosilite series
- Enstatite – MgSiO3
- Ferrosilite – FeSiO3
- Pigeonite – Ca0.25(Mg,Fe)1.75Si2O6
- Diopside – hedenbergite series
- Diopside – CaMgSi2O6
- Hedenbergite – CaFeSi2O6
- Augite – (Ca,Na)(Mg,Fe,Al)(Si,Al)2O6
- Sodium pyroxene series
- Spodumene – LiAlSi2O6
- Enstatite – orthoferrosilite series
- Pyroxenoid group
- Wollastonite – CaSiO3
- Rhodonite – MnSiO3
- Pectolite – NaCa2(Si3O8)(OH)
Double chain inosilicates
- Amphibole group
- Anthophyllite – (Mg,Fe)7Si8O22(OH)2
- Cummingtonite series
- Cummingtonite – Fe2Mg5Si8O22(OH)2
- Grunerite – Fe7Si8O22(OH)2
- Tremolite series
- Tremolite – Ca2Mg5Si8O22(OH)2
- Actinolite – Ca2(Mg,Fe)5Si8O22(OH)2
- Hornblende – (Ca,Na)2–3(Mg,Fe,Al)5Si6(Al,Si)2O22(OH)2
- Sodium amphibole group
- Glaucophane – Na2Mg3Al2Si8O22(OH)2
- Riebeckite (asbestos) – Na2FeII3FeIII2Si8O22(OH)2
- Arfvedsonite – Na3(Fe,Mg)4FeSi8O22(OH)2
 Inosilicate, pyroxene family, with 2-periodic single chain (Si2O6), diopside
Inosilicate, pyroxene family, with 2-periodic single chain (Si2O6), diopside Inosilicate, clinoamphibole, with 2-periodic double chains (Si4O11), tremolite
Inosilicate, clinoamphibole, with 2-periodic double chains (Si4O11), tremolite Inosilicate, unbranched 3-periodic single chain of wollastonite
Inosilicate, unbranched 3-periodic single chain of wollastonite Inosilicate with 5-periodic single chain, rhodonite
Inosilicate with 5-periodic single chain, rhodonite Inosilicate with cyclic branched 8-periodic chain, pellyite
Inosilicate with cyclic branched 8-periodic chain, pellyite
Phyllosilicates
Phyllosilicates (from Greek φύλλον phyllon, leaf), or sheet silicates, form parallel sheets of silicate tetrahedra with Si2O5 or a 2:5 ratio. Nickel–Strunz classification: 09.E. All phyllosilicate minerals are hydrated, with either water or hydroxyl groups attached.

Examples are:
- Serpentine subgroup
- Antigorite – Mg3Si2O5(OH)4
- Chrysotile – Mg3Si2O5(OH)4
- Lizardite – Mg3Si2O5(OH)4
- Clay minerals group
- Halloysite – Al2Si2O5(OH)4
- Kaolinite – Al2Si2O5(OH)4
- Illite – (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2O)]
- Montmorillonite – (Na,Ca)0.33(Al,Mg)2Si4O10(OH)2·nH2O
- Vermiculite – (MgFe,Al)3(Al,Si)4O10(OH)2·4H2O
- Talc – Mg3Si4O10(OH)2
- Sepiolite – Mg4Si6O15(OH)2·6H2O
- Palygorskite (or attapulgite) – (Mg,Al)2Si4O10(OH)·4(H2O)
- Pyrophyllite – Al2Si4O10(OH)2
- Mica group
- Biotite – K(Mg,Fe)3(AlSi3)O10(OH)2
- Fuchsite – K(Al,Cr)2(AlSi3O10)(OH)2
- Muscovite – KAl2(AlSi3)O10(OH)2
- Phlogopite – KMg3(AlSi3)O10(OH)2
- Lepidolite – K(Li,Al)2–3(AlSi3)O10(OH)2
- Margarite – CaAl2(Al2Si2)O10(OH)2
- Glauconite – (K,Na)(Al,Mg,Fe)2(Si,Al)4O10(OH)2
- Chlorite group
- Chlorite – (Mg,Fe)3(Si,Al)4O10(OH)2·(Mg,Fe)3(OH)6
 Phyllosilicate, mica group, muscovite (red: Si, blue: O)
Phyllosilicate, mica group, muscovite (red: Si, blue: O) Phyllosilicate, single net of tetrahedra with 4-membered rings, apophyllite-(KF)-apophyllite-(KOH) series
Phyllosilicate, single net of tetrahedra with 4-membered rings, apophyllite-(KF)-apophyllite-(KOH) series Phyllosilicate, single tetrahedral nets of 6-membered rings, pyrosmalite-(Fe)-pyrosmalite-(Mn) series
Phyllosilicate, single tetrahedral nets of 6-membered rings, pyrosmalite-(Fe)-pyrosmalite-(Mn) series Phyllosilicate, single tetrahedral nets of 6-membered rings, zeophyllite
Phyllosilicate, single tetrahedral nets of 6-membered rings, zeophyllite Phyllosilicate, double nets with 4- and 6-membered rings, carletonite
Phyllosilicate, double nets with 4- and 6-membered rings, carletonite
Tectosilicates
Tectosilicates, or "framework silicates," have a three-dimensional framework of silicate tetrahedra with SiO2 or a 1:2 ratio. This group comprises nearly 75% of the crust of the Earth. Tectosilicates, with the exception of the quartz group, are aluminosilicates. Nickel–Strunz classification: 09.F and 09.G, 04.DA (Quartz/ silica family)
.jpg)
Examples are:
- Quartz group
- Quartz – SiO2
- Tridymite – SiO2
- Cristobalite – SiO2
- Coesite – SiO2
- Stishovite – SiO2
- Moganite – SiO2
- Chalcedony – SiO2
- Feldspar family
- Alkali feldspars (potassium feldspars)
- Microcline – KAlSi3O8
- Orthoclase – KAlSi3O8
- Anorthoclase – (Na,K)AlSi3O8
- Sanidine – KAlSi3O8
- Plagioclase feldspars
- Albite – NaAlSi3O8
- Oligoclase – (Na,Ca)(Si,Al)4O8 (Na:Ca 4:1)
- Andesine – (Na,Ca)(Si,Al)4O8 (Na:Ca 3:2)
- Labradorite – (Ca,Na)(Si,Al)4O8 (Na:Ca 2:3)
- Bytownite – (Ca,Na)(Si,Al)4O8 (Na:Ca 1:4)
- Anorthite – CaAl2Si2O8
- Alkali feldspars (potassium feldspars)
- Feldspathoid family
- Petalite – LiAlSi4O10
- Scapolite group
- Analcime – NaAlSi2O6·H2O
- Zeolite family
Gallery
 Nesosilicate (SiO4)
Nesosilicate (SiO4) Sorosilicate (Si2O7), as in suolunite
Sorosilicate (Si2O7), as in suolunite
[Ca2Si2O5(OH)2·H2O Tectosilicate, aluminosilicate 3D network, zeolite family, synthetic zeolite ZSM-5
Tectosilicate, aluminosilicate 3D network, zeolite family, synthetic zeolite ZSM-5 Silica family (SiO2 3D network), β-quartz
Silica family (SiO2 3D network), β-quartz
See also
Further references
- Deer, W.A.; Howie, R.A.; Zussman, J. (1992). An introduction to the rock-forming minerals (2nd ed.). London: Longman. ISBN 0-582-30094-0.
- Deer, W.A.; Howie, R.A.; Wise, W.S.; Zussman, J. (2004). Rock-forming minerals. Volume 4B. Framework silicates: silica minerals. Feldspathoids and the zeolites (2nd ed.). London: Geological Society of London. p. 982 pp.
- Hurlbut, Cornelius S. (1966). Dana's Manual of Mineralogy (17th ed.). ISBN 0-471-03288-3.
- Hurlbut, Cornelius S.; Klein, Cornelis (1985). Manual of Mineralogy (20th ed.). Wiley. ISBN 0-471-80580-7.
External links
| The Wikibook Historical Geology has a page on the topic of: Silicate minerals |
![]() Media related to Silicates at Wikimedia Commons
Media related to Silicates at Wikimedia Commons

