Phosphatidylcholine transfer protein
| PCTP | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||
| Aliases | PCTP, Pctp, PC-TP, StarD2, phosphatidylcholine transfer protein | ||||||||||||||||||||||||
| External IDs | MGI: 107375 HomoloGene: 32054 GeneCards: PCTP | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez | |||||||||||||||||||||||||
| Ensembl | |||||||||||||||||||||||||
| UniProt | |||||||||||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||||||||||
| RefSeq (protein) | |||||||||||||||||||||||||
| Location (UCSC) | Chr 17: 55.75 – 55.84 Mb | Chr 17: 89.98 – 90 Mb | |||||||||||||||||||||||
| PubMed search | [3] | [4] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Phosphatidylcholine transfer protein (PCTP) also known as StAR-related lipid transfer domain protein 2 (STARD2) is a specific intracellular phospholipid binding protein that can transfer phosphatidylcholine between different membranes in the cytosol.[5][6]
In humans, phosphatidylcholine transfer protein is encoded by the PCTP gene.[7][8]
Function
PCTP transfers phosphatidylcholine molecules between membranes in vitro.[6] Further studies found that sensitivity to phosphatidylcholine levels causes PCTP to interact with select enzymes, promoting their activation. PCTP stimulates the acyl-CoA thioesterase activity of thioesterase superfamily member 2 (Them2)/acyl-CoA thioesterase 13 (ACOT13) and the activity of homeodomain transcription factor paired box gene 3 (PAX3).[9] Protein kinase C phosphorylation promotes localization of PCTP to the mitochondrion where it may activate Them2.[10]
Structure
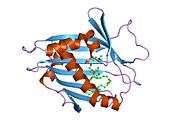
This soluble protein is 214 amino acids long. It is almost entirely composed of a StAR-related transfer domain (START). X-ray crystallography shows that this domain forms a pocket that can bind a single molecule of phosphatidylcholine.[11]
This protein also founds the StarD2 subfamily of proteins. This subfamily consists of PCTP, StarD7, StarD10 and collagen type IV alpha-3-binding protein or StarD11, all of which bind phosphatidylcholine except for StarD11 which prefers ceramide.
Tissue distribution and pathology
PCTP is produced in all tissues in the body at various levels. The protein is expressed at high levels in tissues engaged in high metabolism, notably including the liver and macrophages.[6][12]
No human patients with defects in PCTP have been described to date. Mice lacking PCTP exhibit a resistance to atherosclerosis linked to changes in plasma lipid levels and changes in body weight linked to the level of brown fat use of fatty acids and Them2 activity.[13][14] Loss of PCTP in fasting mice alters the sensitivity of the liver to insulin, reducing glucose and free fatty acid levels.[15]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000141179 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000020553 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ van Golde LM, Oldenborg V, Post M, Batenburg JJ, Poorthuis BJ, Wirtz KW (July 1980). "Phospholipid transfer proteins in rat lung. Identification of a protein specific for phosphatidylglycerol". J. Biol. Chem. 255 (13): 6011–3. PMID 7391000.
- 1 2 3 Wirtz KW (July 1991). "Phospholipid transfer proteins.". Annu. Rev. Biochem. 60 (13): 73–99. PMID 1883207. doi:10.1146/annurev.bi.60.070191.000445.
- ↑ "Entrez Gene: phosphatidylcholine transfer protein".
- ↑ van Helvoort A, de Brouwer A, Ottenhoff R, Brouwers JF, Wijnholds J, Beijnen JH, Rijneveld A, van der Poll T, van der Valk MA, Majoor D, Voorhout W, Wirtz KW, Elferink RP, Borst P (September 1999). "Mice without phosphatidylcholine transfer protein have no defects in the secretion of phosphatidylcholine into bile or into lung airspaces". Proc. Natl. Acad. Sci. U.S.A. 96 (20): 11501–6. PMC 18063
 . PMID 10500206. doi:10.1073/pnas.96.20.11501.
. PMID 10500206. doi:10.1073/pnas.96.20.11501. - ↑ Kanno K, Wu MK, Agate DA, Fanelli BK, Wagle N, Scapa EF, Ukomadu C, Cohen DE (October 2007). "Interacting proteins dictate function of the minimal START domain phosphatidylcholine transfer protein/StarD2". J. Biol. Chem. 282 (42): 30728–36. PMID 17704541. doi:10.1074/jbc.M703745200.
- ↑ de Brouwer AP, Westerman J, Kleinnijenhuis A, Bevers LE, Roelofsen B, Wirtz KW (March 2002). "Clofibrate-induced relocation of phosphatidylcholine transfer protein to mitochondria in endothelial cells". Exp. Cell Res. 274 (1): 100–11. PMID 11855861. doi:10.1006/excr.2001.5460.
- ↑ Roderick SL, Chan WW, Agate DS, Olsen LR, Vetting MW, Rajashankar KR, Cohen DE (July 2002). "Structure of human phosphatidylcholine transfer protein in complex with its ligand". Nature Structural & Molecular Biology. 9 (7): 507–11. PMID 12055623. doi:10.1038/nsb812.
- ↑ Baez JM, Tabas I, Cohen DE (May 2005). "Decreased lipid efflux and increased susceptibility to cholesterol-induced apoptosis in macrophages lacking phosphatidylcholine transfer protein". Biochem. J. 388 (Pt 1): 57–63. PMC 1186693
 . PMID 15628972. doi:10.1042/BJ20041899.
. PMID 15628972. doi:10.1042/BJ20041899. - ↑ Wang WJ, Baez JM, Maurer R, Dansky HM, Cohen DE (2006). "Homozygous disruption of Pctp modulates atherosclerosis in apolipoprotein E deficient mice". J. Lipid. Res. 47 (11): 2400–7. PMID 16940277. doi:10.1194/jlr.M600277-JLR200.
- ↑ Kang HW, Ribich S, Kim BW, Hagen SJ, Bianco AC, Cohen DE (November 2009). "Mice lacking Pctp /StarD2 exhibit increased adaptive thermogenesis and enlarged mitochondria in brown adipose tissue". J. Lipid Res. 50 (11): 2212–21. PMC 2759827
 . PMID 19502644. doi:10.1194/jlr.M900013-JLR200.
. PMID 19502644. doi:10.1194/jlr.M900013-JLR200. - ↑ Scapa EF, Pocai A, Wu MK, Gutierrez-Juarez R, Glenz L, Kanno K, Li H, Biddinger S, Jelicks LA, Rossetti L, Cohen DE (July 2008). "Regulation of energy substrate utilization and hepatic insulin sensitivity by phosphatidylcholine transfer protein/StarD2". FASEB J. 22 (7): 2579–90. PMID 18347010. doi:10.1096/fj.07-105395.