Phenibut
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Noofen, Citrocard |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 64–65%[1][2] |
| Biological half-life | 5.3 hours (250 mg dose)[1] |
| Identifiers | |
| |
| Synonyms | Fenibut, Fenigam, Phenigam, Phenybut, Phenygam, Phenylgamma, PHG, PhGABA[3] |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.012.800 |
| Chemical and physical data | |
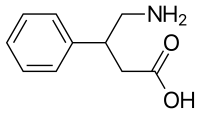
| Formula | C10H13NO2 |
| Molar mass | 179.216 g/mol |
| 3D model (JSmol) | |
| Melting point | 253 °C (487 °F) |
| |
| |
| | |
Phenibut, sold under the brand names Noofen and Citrocard, is a central depressant which is used in Russia for a variety of indications. It has also been reported by some researchers to possess nootropic effects due to its ability to improve neurological functions,[4] but others have not observed these effects.[5] It is generally accepted that phenibut has anxiolytic effects in both animal models and in humans.[1]
Phenibut is chemically related to the neurotransmitter γ-aminobutyric acid (GABA).[1] It is believed to act as a selective GABAB receptor agonist, similarly to baclofen; studies are conflicting as to whether phenibut also acts as a GABAA receptor agonist. More recently, phenibut has been found to act preferentially as a blocker of α2δ subunit-containing voltage-gated calcium channels, similarly to gabapentin and pregabalin.[6][7] As such, by definition, phenibut is a gabapentinoid.[8][9]
Medical uses
Phenibut is used in Russia as a pharmaceutical drug to treat a wide range of ailments, including anxiety, depression, asthenia, insomnia, alcoholism, posttraumatic stress disorder, stuttering, and vestibular disorders, among other conditions.[1][4][10] In many other parts of the world, phenibut is not approved for clinical use, and is instead sold as a nutritional supplement.[11]
Side effects
The side effects of phenibut are said to be similar to but milder than those of baclofen.[10] They include sleepiness and hangover-like effects such as headache and depression once the drug has worn off.[10]
Withdrawal
Limited evidence indicates that withdrawal symptoms of phenibut may include severe anxiety, nervousness, tremors, agitation, dizziness, irritation, fatigue, loss of appetite, rapid heartbeat, nausea, vomiting, tension, psychosis, hallucinations, and insomnia.[10] These symptoms may last as long as two weeks.[10] Tolerance to phenibut may develop rapidly with repeated use.[10]
Overdose
Symptoms of overdose include lowered body temperature, muscle relaxation, and sleepiness.[10]
Interactions
Phenibut should not be combined with alcohol, sedatives, monoamine oxidase inhibitors, anticonvulsants like carbamazepine, or other prescription drugs.[10] Persons on such medications should consult with their medical practitioners prior to taking phenibut. Evidence suggests that phenibut can modulate the function of some epilepsy medications.[4]
Pharmacology
Phenibut acts as an agonist of the GABAB receptor (specifically, a full agonist),[12] similar to baclofen and γ-hydroxybutyric acid (GHB),[13] and at higher doses also of the GABAA receptor.[4][14] It has some 30- to 68-fold lower affinity for the GABAB receptor relative to baclofen, which is active at far lower doses in comparison.[12] There is dispute in the literature about whether or not phenibut binds to the GABAA receptor, which is the receptor responsible for the actions of the benzodiazepines, barbiturates, and Z-drugs, and for the main effects of ethanol. According to Allikmets and Ryage (1983) and Shulgina (1986), phenibut does bind to the GABAA receptor,[4] but according to Lapin (2001), it does not.[1] In the case of the former, it is argued that the GABAA binding only occurs at higher concentrations.[4]
Subsequent research has found that phenibut also binds to and potently blocks α2δ subunit-containing voltage-gated calcium channels (VGCCs), similarly to gabapentin and pregabalin, and hence is a gabapentinoid.[6][7] Both enantiomers of phenibut show this action with similar efficacy.[6] Moreover, the R-enantiomer possesses five-fold greater affinity for this site relative to the GABAB receptor, while the S-enantiomer does not bind to the GABAB receptor at all.[6] As such, phenibut is likely to have much greater effect on α2δ subunit-containing VGCCs than on the GABAB receptor.[6] Notably, the antinociceptive effects of phenibut in rodents are mediated not by the GABAB receptor but by blockade of α2δ subunit-containing VGCCs.[6]
The literature that supports the nootropic effects of phenibut also suggest that it elicits tranquilizing effects,[1] reduction of stress and anxiety, improvement of impaired sleep, and the potentiation (enhancement) of the effects of tranquilizers, narcotics, and neuroleptics. It is also suggested to have an anticonvulsant effect,[4] though studies on other GABAB agonists, such as GHB and the phenibut analogue baclofen, have shown them to act as potential convulsants. It should be noted, however, that GHB acts on the convulsion-inducing GHB receptor,[15] which phenibut does not. Phenibut has been found to reduce immobility time in the forced swim test, a measure of possible antidepressant activity, which was blocked by a GABAB receptor antagonist.[12] It has been found to increase dopamine levels in the striatum,[1] and it was suggested that phenibut may activate dopaminergic processes and that this may be involved in its effects.[1] Anecdotal evidence indicates that phenibut can produce euphoria.[10] Recreational use of the drug has been reported.[16] In Europe, phenibut has been mentioned as a "novel psychoactive substance",[17] although it is produced and prescribed in an EU member Latvia, with its producer Olainfarm recently receiving EU funding for expanding facilities used for phenibut's intermediate product production.[18]
Chemistry
Phenibut is a derivative of the inhibitory neurotransmitter GABA with a phenyl group in the β-position. Its name is a contraction of its chemical name β-phenyl-γ-aminobutyric acid (β-phenyl-GABA). As such, it is a GABA analogue, and is closely related to certain other GABA analogues like baclofen (β-(4-chlorophenyl)-GABA), pregabalin (β-isobutyl-GABA), GABOB (β-hydroxy-GABA), and tolibut (β-(4-methylphenyl)-GABA). Unlike GABA, the presence of the phenyl ring allows phenibut to cross the blood–brain barrier.[1] It has almost the same chemical structure as baclofen, lacking only a chlorine atom in the para-position of the phenyl ring,[4] and also contains phenethylamine in its structure.[1] The structure of phenibut is also similar to that of pregabalin, in which the phenyl ring is substituted with an isobutyl group.
Phenibut is a chiral molecule and thus has two potential configurations, as (R)- and (S)-enantiomers.[13] In its hydrochloride salt form, it is a white crystalline powder with a very sour taste. It is readily soluble in water and in alcohol, and the pH of a 2.5% water solution is about 2.3–2.7.
History
Phenibut was synthesized at the A. I. Herzen Leningrad Pedagogical Institute (USSR) by Professor Vsevolod Perekalin's team and tested at the Institute of Experimental Medicine, USSR Academy of Medical Sciences.
Phenibut is mandated standard equipment in a Russian cosmonaut's medical kit. The use of "conventional" tranquilizers for stress and anxiety makes patients drowsy, which was deemed unacceptable for cosmonauts; phenibut, however, lowers stress levels without adversely affecting performance. In 1975, phenibut was included in the cosmonauts' kit for those who participated in the Apollo-Soyuz joint mission.[19]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 Lapin I (2001). "Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug". CNS Drug Rev. 7 (4): 471–81. PMID 11830761.
- ↑ Smirnova LA, Perfilova VN, Tyurenkov IN, Ryabukha AF, Suchkov EA, Lebedeva SA (2013). "Study of the absolute bioavailability of citrocard, a new GABA derivative". Bull. Exp. Biol. Med. 155 (4): 458–60. PMID 24143367. doi:10.1007/s10517-013-2177-2.
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 69–. ISBN 978-1-4757-2085-3.
- 1 2 3 4 5 6 7 8 Shulgina GI (1986). "On neurotransmitter mechanisms of reinforcement and internal inhibition". Pavlov J Biol Sci. 21 (4): 129–40. PMID 2431377.
- ↑ Kovaleva EL (1984). "[Comparative characteristics of the nootropic action of fenibut and fepiron]". Farmakol Toksikol (in Russian). 47 (1): 20–3. PMID 6705902.
- 1 2 3 4 5 6 Zvejniece, Liga; Vavers, Edijs; Svalbe, Baiba; Veinberg, Grigory; Rizhanova, Kristina; Liepins, Vilnis; Kalvinsh, Ivars; Dambrova, Maija (2015). "R-phenibut binds to the α2–δ subunit of voltage-dependent calcium channels and exerts gabapentin-like anti-nociceptive effects". Pharmacology Biochemistry and Behavior. 137: 23–29. ISSN 0091-3057. PMID 26234470. doi:10.1016/j.pbb.2015.07.014.
- 1 2 Vavers, Edijs; Zvejniece, Liga; Svalbe, Baiba; Volska, Kristine; Makarova, Elina; Liepinsh, Edgars; Rizhanova, Kristina; Liepins, Vilnis; Dambrova, Maija (2015). "The neuroprotective effects of R-phenibut after focal cerebral ischemia". Pharmacological Research. ISSN 1043-6618. doi:10.1016/j.phrs.2015.11.013.
- ↑ Elaine Wyllie; Gregory D. Cascino; Barry E. Gidal; Howard P. Goodkin (17 February 2012). Wyllie's Treatment of Epilepsy: Principles and Practice. Lippincott Williams & Wilkins. p. 423. ISBN 978-1-4511-5348-4.
- ↑ Honorio Benzon; James P. Rathmell; Christopher L. Wu; Dennis C. Turk; Charles E. Argoff; Robert W Hurley (11 September 2013). Practical Management of Pain. Elsevier Health Sciences. p. 1006. ISBN 978-0-323-17080-2.
- 1 2 3 4 5 6 7 8 9 David W. Group (25 February 2015). Encyclopedia of Mind Enhancing Foods, Drugs and Nutritional Substances, 2d ed. McFarland. pp. 186–. ISBN 978-0-7864-4142-6.
- ↑ Nelson, LS (2008). "Phenibut Withdrawal - A Novel 'Nutritional Supplement'". Clinical Toxicology. 46 (7): 605. doi:10.1080/15563650802255033.
- 1 2 3 GABAb Receptor Pharmacology: A Tribute to Norman Bowery: A Tribute to Norman Bowery. Academic Press. 21 September 2010. pp. 25–. ISBN 978-0-12-378648-7.
- 1 2 Dambrova M, Zvejniece L, Liepinsh E, Cirule H, Zharkova O, Veinberg G, Kalvinsh I (2008). "Comparative pharmacological activity of optical isomers of phenibut". Eur. J. Pharmacol. 583 (1): 128–34. PMID 18275958. doi:10.1016/j.ejphar.2008.01.015.
- ↑ Zyablitseva, Evgeniya A.; Kositsyn, Nikolay S.; Shul'gina, Galina I. (2013). "The Effects of Agonists of Ionotropic GABAA and Metabotropic GABAB Receptors on Learning". The Spanish journal of psychology. 12 (01): 12–20. ISSN 1138-7416. doi:10.1017/S1138741600001438.
- ↑ Banerjee PK, Snead OC (1995). "Presynaptic gamma-hydroxybutyric acid (GHB) and gamma-aminobutyric acidB (GABAB) receptor-mediated release of GABA and glutamate (GLU) in rat thalamic ventrobasal nucleus (VB): a possible mechanism for the generation of absence-like seizures induced by GHB". J. Pharmacol. Exp. Ther. 273 (3): 1534–43. PMID 7791129.
- ↑ Wong A, Little M, Caldicott D, Easton C, Andres D, Greene SL (2015). "Analytically confirmed recreational use of Phenibut (β-phenyl-γ-aminobutyric acid) bought over the internet". Clin Toxicol (Phila). 53 (7): 783–4. PMID 26107626. doi:10.3109/15563650.2015.1059944.
- ↑ David R. Owen; David M. Wood; John R. H. Archer; Paul I. Dargan (December 2015). "Phenibut (4-amino-3-phenyl-butyric acid): Availability, prevalence of use, desired effects and acute toxicity". Drug and Alcohol Review. doi:10.1111/dar.12356.
- ↑ BNS (11 September 2015). "Olainfarm investē teju divus miljonus eiro zāļu ražošanas iecirkņa modernizācijā" [Olainfarm invests nearly 2 million euros in an upgrade of a drug production unit] (in Latvian). Retrieved 16 January 2016.
- ↑ Slava Lapin (30 July 2009). From the Inside. Luniver Press. p. 209. ISBN 978-1-905986-11-8. Retrieved 6 November 2010.
External links
- 4-Amino-3-phenylbutyric acid in the ChemIDplus database