Peripatric speciation
| Part of a series on |
| Evolutionary biology |
|---|
 |
|
History of evolutionary theory |
|
Fields and applications
|
|
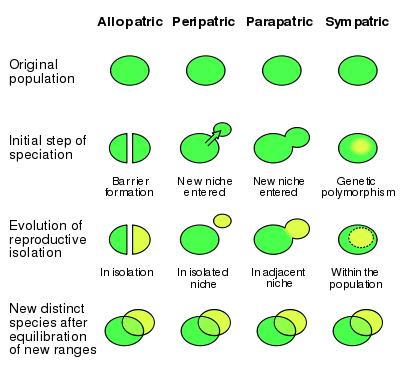
Peripatric speciation is a mode of speciation in which a new species is formed from an isolated peripheral population.[1] Since peripatric speciation resembles allopatric speciation, in that populations are isolated and prevented from exchanging genes, it can often be difficult to distinguish between them.[2] Nevertheless, the primary characteristic of peripatric speciation proposes that one of the populations is much smaller than the other.
The terms peripatric and peripatry are often used in biogeography, referring to organisms whose ranges are closely adjacent but do not overlap, being separated where these organisms do not occur—for example on an oceanic island compared to the mainland. Such organisms are usually closely related (e.g. sister species); their distribution being the result of peripatric speciation. An alternative model of peripatric speciation, centrifugal speciation, posits that a species' population experiences periods of geographic range expansion followed by shrinking periods, leaving behind small isolated populations on the periphery of the main population. Other models have involved the effects of sexual selection on limited population sizes.
The existence of peripatric speciation is supported by laboratory experiments and empirical evidence.[1] Scientists observing the patterns of a species biogeographic distribution and its phylogenetic relationships are able to reconstruct the historical process by which they diverged. Further, oceanic islands are often the subject of peripatric speciation research due to their isolated habitats—with the Hawaiian Islands widely represented in much of the scientific literature.
History
Peripatric speciation was first proposed by Ernst Mayr in 1954[3] and fully theoretically modeled in 1982.[4] It is related to the founder effect, where small living populations may undergo selection bottlenecks.[5] The founder effect is based on models that suggest peripatric speciation can occur by the interaction of selection and genetic drift,[1] which may play a significant role.[6] In 1976 and 1980, the Kaneshiro model of peripatric speciation was developed by Kenneth Y. Kaneshiro which focused on sexual selection as a driver for speciation during population bottlenecks.[7][8][9]
Models
Peripatric
Peripatric speciation models are identical to models of vicariance (allopatric speciation).[1] Requiring both geographic separation and time, speciation can result as a predictable byproduct.[10] Peripatry can be distinguished from allopatric speciation by three key features:[1]
- The size of the isolated population
- Strong selection caused by the dispersal and colonization of novel environments,
- The effects of genetic drift on small populations.
The size of a population is important because individuals colonizing a new habitat likely contain only a small sample of the genetic variation of the original population. This promotes divergence due to strong selective pressures, leading to the rapid fixation of an allele within the descendant population. This gives rise to the potential for genetic incompatibilities to evolve. These incompatibilities cause reproductive isolation, giving rise to—sometimes rapid—speciation events.[1] Furthermore, two important predictions are invoked, namely that geological or climactic changes cause populations to become locally fragmented (or regionally when considering allopatric speciation), and that an isolated population's reproductive traits evolve enough as to prevent interbreeding upon potential secondary contact.[11]
The peripatric model results in, what have been called, progenitor-derivative species pairs, whereby the derivative species (the peripherally isolated population)—geographically and genetically isolated from the progenitor species—diverges.[12] A specific phylogenetic signature results from this mode of speciation. That is, the central population remains pleisomorphic, while the peripheral isolates become apomorphic.[13]
One possible consequence of peripatric speciation is that a geographically widespread ancestral species becomes paraphyletic, thereby becoming a paraspecies.[14] The concept of a paraspecies is therefore a logical consequence of the evolutionary species concept, by which one species gives rise to a daughter species.[15]
.png)
.png)
Centrifugal speciation
William Louis Brown, Jr. proposed an alternative model of peripatric speciation in 1957 called centrifugal speciation. This model contrasts with peripatric speciation by virtue of the origin of the genetic novelty that leads to reproductive isolation.[16] A population of a species experiences periods of geographic range expansion followed by periods of contraction. During the contraction phase, fragments of the population become isolated as small refugial populations on the periphery of the central population (see figure 2b). Because of the large size and potentially greater genetic variation within the central population, mutations arise more readily. These mutations are left in the isolated peripheral populations, whereby, promoting reproductive isolation. Consequently, Brown suggested that during another expansion phase, the central population would overwhelm the peripheral populations, hindering speciation. However, if the species finds a specialized ecological niche, the two may coexist.[17][18] The phylogenetic signature of this model is that the central population becomes derived, while the peripheral isolates stay pleisomorphic[13]—the reverse of the general model.
Centrifugal speciation has been largely ignored in the scientific literature, often dominated by the traditional model of peripatric speciation.[19][16][13] Despite this, Brown cited a wealth of evidence to support his model, of which has not yet been refuted.[17]
Peromyscus polionotus and P. melanotis (the peripherally isolated species from the central population of P. maniculatus) arose via the centrifugal speciation model.[20] Centrifugal speciation may have taken place in tree kangaroos, South American frogs (Ceratophrys), shrews (Crocidura), and primates (Presbytis melalophos).[19] John C. Briggs associates centrifugal speciation with centers of origin, contending that the centrifugal model is better supported by the data, citing species patterns from the proposed 'center of origin' within the Indo-West Pacific[19]
Kaneshiro model
When a sexual species experiences a population bottleneck—that is, when the genetic variation is reduced due to small population size—mating discrimination among females may be altered by the decrease in courtship behaviors of males.[9] Sexual selection pressures may become weakened by this in an isolated peripheral population, and as a by-product of the altered mating recognition system, secondary sexual traits may appear.[7] Eventually, a growth in population size paired with novel female mate preferences will give rise to reproductive isolation from the main population-thereby completing the peripatric speciation process.[8]
Support for this model comes from experiments and observation of species that exhibit asymmetric mating patterns such as the Hawaiian Drosophila species[21][22] or the Hawaiian cricket Laupala.[23] However, this model has not been entirely supported by experiments, and therefore, it may not represent a plausible process of peripatric speciation that takes place in nature.[9]
Evidence
Species patterns on islands and archipelagos
Island species provide direct evidence of speciation occurring peripatrically in such that, "the presence of endemic species on oceanic islands whose closest relatives inhabit a nearby continent" must have originated by a colonization event.[1] Comparative phylogeography of oceanic archipelagos shows consistent patterns of sequential colonization and speciation along island chains, most notably on the Azores islands, Canary Islands, Society Islands, Marquesas Islands, Galápagos Islands, Austral Islands, and the Hawaiian Islands—all of which express geological patterns of spatial isolation and, in some cases, linear arrangement.[24]
Hawaiian archipelago
.png)
.png)
Drosophila species on the Hawaiian archipelago have helped researchers understand speciation processes in great detail. It is well established that Drosophila has undergone an adaptive radiation into hundreds of endemic species on the Hawaiian island chain;[1][25] originating from a single common ancestor (supported from molecular analysis).[26] Studies consistently find that colonization of each island occurred from older to younger islands, and in Drosophila, speciating peripatrically at least fifty percent of the time.[1] In conjunction with Drosophila, Hawaiian lobeliads (Cyanea) have also undergone an adaptive radiation, with upwards of twenty-seven percent of extant species arising after new island colonization—exemplifying peripatric speciation—once again, occurring in the old-to-young island direction.[27][28][29] (See figure 3a).
Other endemic species in Hawaii also provide evidence of peripatric speciation such as the endemic flightless crickets (Laupala). It has been estimated that, "17 species out of 36 well-studied cases of [Laupala] speciation were peripatric".[1][30] Plant species in genera's such as Dubautia, Wilkesia, and Argyroxiphium have also radiated along the archipelago.[31]
Tetragnatha spiders have also speciated peripatrically on the Hawaiian islands,[32][33][1] Numerous arthropods have been documented existing in patterns consistent with the geologic evolution of the island chain, in such that, phylogenetic reconstructions find younger species inhabiting the geologically younger islands and older species inhabiting the older islands (or in some cases, ancestors date back to when islands currently below sea level were exposed). Spiders such as those from the genus Orsonwelles exhibit patterns compatible with the old-to-young geology.[34] Other endemic genera such as Argyrodes have been shown to have speciated along the island chain.[35] Pagiopalus, Pedinopistha, and part of the Thomisidae family have adaptively radiated along the island chain,[36] as well as the Lycosidae family of wolf spiders.[37]
A host of other Hawaiian endemic arthropod species and genera have had their speciation and phylogeographical patterns studied: the Drosophila grimshawi species complex,[38] damselflies (Megalagrion xanthomelas and Megalagrion pacificum),[39] Doryonychus raptor, Littorophiloscia hawaiiensis, Anax strenuus, Nesogonia blackburni, Theridion grallator[40] (see figure 3b), Vanessa tameamea, Hyalopeplus pellucidus, Coleotichus blackburniae, Labula, Hawaiioscia, Banza (in the Tettigoniidae family), Caconemobius, Eupethicea, Ptycta, Megalagrion, Prognathogryllus, Nesosydne, Cephalops, Trupanea, and the tribe Platynini—all suggesting repeated radiations among the islands.[41]
Other animals besides insects show this same pattern such as the Hawaiian amber snail (Succinea caduca),[42] and ‘Elepaio flycatchers.[43]
Other islands
Phylogenetic studies of a species of crab spider (Misumenops rapaensis) in the genus Thomisidae located on the Austral Islands have established the, "sequential colonization of [the] lineage down the Austral archipelago toward younger islands". Interestingly, M. rapaensis has been traditionally thought of as a single species; whereas this particular study found distinct genetic differences corresponding to the sequential age of the islands.[44]
Species patterns on continents
.png)
The occurrence of peripatry on continents is more difficult to detect due to the possibility of vicariant explanations being equally likely.[1] However, studies concerning the Californian plant species Clarkia biloba and C. biloba strongly suggest a peripatric origin.[45] In addition, a great deal of research has been conducted on several species of land snails involving chirality that suggests peripatry (with some authors noting other possible interpretations).[1]
The chestnut-tailed antbird is located within the Serrania de Huanchaca in Bolivia. Within this region exists an fringe patch of forest (see figure 3c) estimated to have been isolated for 1000–3000 years. The population of antbirds that reside in the isolated patch express significant song divergence; thought to be an "early step" in the process of peripatric speciation. Further, peripheral isolation "may partly explain the dramatic diversification of suboscines in Amazonia".[11]
A study by Lucinda P. Lawson et al. found evidence for the occurrence of peripatric speciation in the montane spiny throated reed frog species complex (genus: Hyperolius). Lawson maintains that the species' geographic ranges within the Eastern Afromontane Biodiversity Hotspot support a peripatric model that is driving speciation; suggesting that this mode of speciation may play a significant role in "highly fragmented ecosystems".[2]
In a study of the phylogeny and biogeography of the land snail genus Monacha, the species M. ciscaucasica is thought to have speciated peripatrically from a population of M. roseni. In addition, M. claussi consists of a small population located on the peripheral of the much larger range of M. subcarthusiana suggesting that it also arose by peripatric speciation.[46]
Red spruce (Picea rubens) has arisen from an isolated population of black spruce (Picea mariana). During the Pleistocene, a population of black spruce became geographically isolated, likely due to glaciation. The geographic range of the black spruce is much larger than the red spruce. The red spruce has significantly lower genetic diversity in both its DNA and its mitochondrial DNA than the black spruce.[47][48] Furthermore, the genetic variation of the red spruce has no unique mitochondrial haplotypes, only subsets of those in the black spruce; suggesting that the red spruce speciated peripatrically from the black spruce population.[49][50][51] It is thought that the entire Picea genus in North America has been diversified by the process of peripatric speciation, as numerous pairs of closely related species in the genus have smaller southern population ranges; and those with overlapping ranges often exhibit weak reproductive isolation.[52][48]
Using a phylogeographic approach paired with ecological niche models (i.e. prediction and identification of expansion and contraction species ranges into suitable habitats based on current ecological niches, correlated with fossil and molecular data), researchers found that the prairie dog species Cynomys mexicanus speciated peripatrically from Cynomys ludovicianus approximately 230,000 years ago. North American glacial cycles promoted range expansion and contraction of the prairie dogs, leading to the isolation of a relic population in a refugium located in the present day Coahuila, Mexico.[53] This distribution and paleobiogeographic pattern correlates with other species expressing similar biographic range patterns[53] such as with the Sorex cinereus complex.[54]
Laboratory experiments
Peripatric speciation has been researched in both laboratory studies and nature. Coyne and Orr in Speciation suggest that most laboratory studies of allopatric speciation are also examples of peripatric speciation due to their small population sizes and the inevitable divergent selection that they undergo.[1]
Much of the laboratory research concerning peripatry is inextricably linked to founder effect research. Coyne and Orr conclude that selection's role in speciation is well established, whereas genetic drift's role is unsupported by experimental and field data—suggesting that founder-effect speciation does not occur.[55] Nevertheless, a great deal of research has been conducted on the matter, and one study conducted involving bottleneck populations of Drosophila pseudoobscura found evidence of isolation after a single bottleneck.[56][57]
Below is a non-exhaustive table of laboratory experiments focused explicitly on peripatric speciation. Most of the studies also conducted experiments on vicariant speciation as well. The "replicates" column signifies the number of lines used in the experiment—that is, how many independent populations were used (not the population size or the number of generations performed).[9]
| Species | Replicates | Reference | Year |
|---|---|---|---|
| Drosophila adiastola | 1 | [58] | 1979 |
| Drosophila silvestris | 1 | [59] | 1980 |
| Drosophila pseudoobscura | 8 | [60] | 1985 |
| Drosophila simulans | 8 | [61] | 1985 |
| Musca domestica | 6 | [62] | 1991 |
| Drosophila pseudoobscura | 42 | [63] | 1993 |
| Drosophila melanogaster | 50 | [64] | 1998 |
| Drosophila melanogaster | 19; 19 | [65] | 1999 |
| Drosophila grimshawi | 1 | [9] | N/A |
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Jerry A. Coyne and H. Allen Orr (2004), Speciation, Sinauer Associates, pp. 105–111, ISBN 0-87893-091-4
- 1 2 Lucinda P. Lawson; et al. (2015), "Divergence at the edges: peripatric isolation in the montane spiny throated reed frog complex", BMC Evolutionary Biology, 15 (128), doi:10.1186/s12862-015-0384-3
- ↑ Mayr, E. 1954. Change of genetic environment and evolution. In: Evolution as a Process (J. Huxley, A. C. Hardy & E. B. Ford, eds), pp. 157–180. Unwin Brothers, London.
- ↑ Mayr, E. 1982. Processes of speciation in animals. In: Mechanisms of Speciation (A. R. I. Liss, ed.), pp. 1–19. Alan R. Liss Inc., New York.
- ↑ Provine WB (1 July 2004). "Ernst Mayr: Genetics and speciation". Genetics. 167 (3): 1041–6. PMC 1470966
 . PMID 15280221.
. PMID 15280221. - ↑ Templeton AR (1 April 1980). "The theory of speciation via the founder principle". Genetics. 94 (4): 1011–38. PMC 1214177
 . PMID 6777243.
. PMID 6777243. - 1 2 Kenneth Y. Kaneshiro (1976), "Ethological isolation and phylogeny in the Plantibia subgroup of Hawaian Drosophila", Evolution, 30 (4): 740–745, PMID 28563322, doi:10.1111/j.1558-5646.1976.tb00954.x
- 1 2 Kenneth Y. Kaneshiro (1980), "Sexual selection, speciation and the direction of evolution", Evolution, 34 (3): 437–444, PMID 28568697, doi:10.1111/j.1558-5646.1980.tb04833.x
- 1 2 3 4 5 Anders Ödeen and Ann-Britt Florin (2002), "Sexual selection and peripatric speciation: the Kaneshiro model revisited", Journal of Evolutionary Biology, 15: 301–306
- ↑ Michael Turelli, Nicholas H. Barton, and Jerry A. Coyne (2001), "Theory and speciation", Trends in Ecology & Evolution, 16 (7): 330–343
- 1 2 Nathalie Seddon and Joseph A. Tobias (2007), "Song divergence at the edge of Amazonia: an empirical test of the peripatric speciation model", Biological Journal of the Linnean Society, 90: 173–188
- ↑ Daniel J. Crawford (2010), "Progenitor-derivative species pairs and plant speciation", Taxon, 59 (5): 1413–1423
- 1 2 3 Jennifer K. Frey (1993), "Modes of Peripheral Isolate Formation and Speciation", Systematic Biology, 42 (3): 373–381
- ↑ Jerry A Coyne and H. Allen Orr (2004), Speciation, Sinauer Associates, p. 470, ISBN 0-87893-091-4
- ↑ Albert, James S.; Reis, Roberto E. (2011). Historical Biogeography of Neotropical Freshwater Fishes. ISBN 978-0-520-26868-5.
- 1 2 Sergey Gavrilets; et al. (2000), "Patterns of Parapatric Speciation", Evolution, 54 (4): 1126–1134
- 1 2 Howard, D. J. 2003. Speciation: Allopatric. eLS
- ↑ W. L. Brown Jr. (1957), "Centrifugal speciation", Quarterly Review of Biology, 32 (3): 247–277
- 1 2 3 John C. Briggs (2000), "Centrifugal speciation and centres of origin", Journal of Biogeography, 27: 1183 –1188
- ↑ Ira F. Greenbaum, Robert J. Baker and Paul R. Ramsey (1978), "Chromosomal Evolution and the Mode of Speciation in Three Species of Peromyscus", Evolution, 32 (3): 646–654, PMID 28567964, doi:10.1111/j.1558-5646.1978.tb04609.x
- ↑ Kenneth Y. Kaneshiro (1983), "Sexual selection and direction of evolution in the biosystematics of Hawaiian Drosophilidae", Annual Review of Entomology, 28: 161–178
- ↑ Luther Val Giddings and Alan R. Templeton (1983), "Behavioral Phylogenies and the Direction of Evolution", Science, 220 (4595): 372–378, doi:10.1126/science.220.4595.372
- ↑ Kerry L. Shaw and Ezequiel Lugo (2001), "Mating asymmetry and the direction of evolution in the Hawaiian cricket genus Laupala", Molecular Ecology, 10 (3): 751–759, doi:10.1046/j.1365-294x.2001.01219.x
- ↑ Kerry L. Shawa and Rosemary G. Gillespie (2016), "Comparative phylogeography of oceanic archipelagos: Hotspots for inferences of evolutionary process", PNAS, 113 (29): 7986–7993, doi:10.1073/pnas.1601078113
- ↑ Hannes Schuler, Glen R. Hood, Scott P. Egan, and Jeffrey L. Feder (2016), "Modes and Mechanisms of Speciation", Rev. Cell Biol. Mol. Medicine, 2 (3): 60–93
- ↑ DeSalle R. (1995). Molecular approaches to biogeographic analysis of Hawaiian Drosophilidae. Pp. 72-89 in W.L. Wagner and V.A. Funk (eds.) Hawaiian Biogeography: Evolution on a Hot-Spot Archipeligo. Smithsonian Institution Press, Washington DC.
- ↑ Givnish, T. J. (1998). Adaptive plant evolution on islands: classical patterns, molecular data, new insights. Evolution on islands, 281, 304.
- ↑ Givnish, T. J., et al. (1995). Molecular evolution, adaptive radiation, and geographic speciation in Cyanea (Campanulaceae, Lobeliodeae). Pp. 259-301 in W.L. Wagner and V.A. Funk (eds.) Hawaiian Biogeography: Evolution on a Hot-Spot Archipeligo. Smithsonian Institution Press, Washington DC.
- ↑ Thomas J. Givnish1, Kendra C. Millam, Austin R. Mast, Thomas B. Paterson, Terra J. Theim, Andrew L. Hipp, Jillian M. Henss, James F. Smith, Kenneth R. Wood, and Kenneth J. Sytsma (2009), "Origin, adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae)", Proc. R. Soc. B, 276: 407–416
- ↑ Kerry L. Shaw (2002), "Conflict between nuclear and mitochondrial DNA phylogenies of a recent species radiation: What mtDNA reveals and conceals about modes of speciation in Hawaiian crickets", PNAS, 99 (25)
- ↑ Martha S. Witter (1990), "Evolution in the Madiinae: Evidence from Enzyme Electrophoresis", Annals of the Missouri Botanical Garden, 77 (1): 110–117
- ↑ Gillespie, R. G. and H. B. Croom (1995). Comparison of speciation mechanisms in web-building and non-web-building groups within a lineage of spiders. Pp. 121-146 in W.L. Wagner and V.A. Funk (eds.) Hawaiian Biogeography: Evolution on a Hot-Spot Archipeligo. Smithsonian Institution Press, Washington DC.
- ↑ Rosemary G. Gillespie (2005), "Geographical context of speciation in a radiation of Hawaiian Tetragnatha spiders (Aranae, Tetragnathidae", The Journal of Arachnology, 33: 313–322
- ↑ Gustavo Hormiga, Miquel Arnedo, and Rosemary Gillespie (2003), "Speciation on a Conveyor Belt: Sequential Colonization of the Hawaiian Islands by Orsonwelles Spiders (Araneae, Linyphiidae)", Systematic Biology, 52 (1): 70–88, PMID 12554442, doi:10.1080/10635150390132786
- ↑ Rosemary G. Gillespie, Malia A. J. Rivera, and Jessica E. Garb. (1998). Sun, surf and spiders: taxonomy and phylogeography of Hawaiian Araneae. Proceedings of the 17th European Colloquium of Arachnology.
- ↑ Jessica E. Garb (1999), "An Adaptive Radiation of Hawaiian Thomisidae: Biogreographic and Genetic Evidence", The Journal of Arachnology, 27: 71–78
- ↑ W. J. Gertsch (1973), "The cavernicolous fauna of Hawaiian lava tubes. 3. Araneae (Spiders)", Pacific Insects, 15: 163–180
- ↑ Fabio Piano, Elysse M. Craddock, and Michael P. Kambysellis (1997), "Phylogeny of the Island Populations of the Hawaiian Drosophila grimshawi Complex: Evidence from Combined Data", Molecular Phylogenetics and Evolution, 7 (2): 173–184
- ↑ Steve Jordan, Chris Simon, David Foote, and Ronald A. Englund (2005), "Phylogeographic patterns of Hawaiian Megalagrion damselflies (Odonata: Coenagrionidae) correlate with Pleistocene island boundaries", Molecular Ecology, 14 (11): 3457–3470, doi:10.1111/j.1365-294X.2005.02669.x
- ↑ Peter J. P. Croucher, Geoff S. Oxford, Athena Lam, Neesha Mody, and Rosemary G. Gillespie (2012), "COLONIZATION HISTORY AND POPULATION GENETICS OF THE COLOR-POLYMORPHIC HAWAIIAN HAPPY-FACE SPIDER THERIDION GRALLATOR (ARANEAE, THERIDIIDAE)", Evolution, 66 (9): 2815–2833, doi:10.1111/j.1558-5646.2012.01653.x
- ↑ G. K. Roderick and R. G. Gillespie (1998), "Speciation and phylogeography of Hawaiian terrestrial arthropods", Molecular Ecology, 7: 519–531
- ↑ Brenden S. Holland and Robert H. Cowie (2007), "A geographic mosaic of passive dispersal: population structure in the endemic Hawaiian amber snail Succinea caduca (Mighels, 1845)", Molecular Ecology, 16 (12): 2422–2435, doi:10.1111/j.1365-294X.2007.03246.x
- ↑ Eric A. VanderWerf, Lindsay C. Young, Norine W. Yeung, and David B. Carlon (2010), "Stepping stone speciation in Hawaii's flycatchers: molecular divergence supports new island endemics within the elepaio", Conservation Genetics, 11 (4): 1283–1298, doi:10.1007/s10592-009-9958-1
- ↑ Jessica E. Garb and Rosemary G. Gillespie (2006), "Island hopping across the central Pacific: mitochondrial DNA detects sequential colonization of the Austral Islands by crab spiders (Araneae: Thomisidae)", Journal of Biogeography, 33 (2): 201–220, doi:10.1111/j.1365-2699.2005.01398.x
- ↑ H. Lewis and M. R. Roberts (1956), "The origin of Clarkia lingulata", Evolution, 10: 126–138
- ↑ Marco T. Neiber and Bernhard Hausdorf (2016), "Molecular phylogeny and biogeography of the land snail genus Monacha (Gastropoda, Hygromiidae)", Zoologica Scripta, 46 (3): 1–14, doi:10.1111/zsc.12218
- ↑ Gary J. Hawley and Donald H. DeHayes (1994), "Genetic diversity and population structure of red spruce (Picea rubens)", Canadian Journal of Botany, 72 (12): 1778–1786, doi:10.1139/b94-219
- 1 2 Juan P. Jaramillo-Correa and Jean Bousquet (2003), "New evidence from mitochondrial DNA of a progenitor-derivitive species relationship between black and red spruce (Pinaceae)", American Journal of Botany, 90 (12): 1801–1806
- ↑ J. P. Jaramillo-Correa, J. Bousquet, J. Beaulieu, N. Isabel, M. Perron, and M. Bouillé (2003), "Cross-species amplification of mitochondrial DNA sequence-tagged-site markers in conifers: the nature of polymorphism and variation within and among species in Picea", Theoretical and Applied Genetics, 106 (8): 1353–1367, doi:10.1007/s00122-002-1174-z
- ↑ Isabelle Gamache, Juan P. Jaramillo-Correa, Sergey Payette, and Jean Bousquet (2003), "Diverging patterns of mitochondrial and nuclear DNA diversity in subarctic black spruce: imprint of a founder effect associated with postglacial colonization", Molecular Ecology, 12 (4): 891–901, PMID 12753210
- ↑ Martin Perron, Daniel J. Perry, Christophe Andalo, and Jean Bousquet (2000), "Evidence from sequence-tagged-site markers of a recent progenitor-derivative species pair in conifers", PNAS, 97 (21): 11331–11336
- ↑ Wright, J. W. (1955), "Species crossability in Spruce in relation to distribution and taxonomy", Forest Science, 1 (4): 319–349
- 1 2 Gabriela Castellanos-Morales, Niza Gámez, Reyna A. Castillo-Gámez, and Luis E. Eguiarte (2016), "Peripatric speciation of an endemic species driven by Pleistocene climate change: The case of the Mexican prairie dog (Cynomys mexicanus)", Molecular Phylogenetics and Evolution, 94: 171–181, doi:10.1016/j.ympev.2015.08.027
- ↑ Andrew G. Hope, Kelly A. Speer, John R. Demboski, Sandra L. Talbot, and Joseph A. Cook (2012), "A climate for speciation: Rapid spatial diversification within the Sorex cinereus complex of shrews", Molecular Phylogenetics and Evolution, 64 (3): 671–684, doi:10.1016/j.ympev.2012.05.021
- ↑ Jerry A. Coyne and H. Allen Orr (2004), Speciation, Sinauer Associates, p. 410, ISBN 0-87893-091-4
- ↑ Jeffrey R. Powell (1978), "The Founder-Flush Speciation Theory: An Experimental Approach", Evolution, 32 (3): 465–474, PMID 28567948, doi:10.1111/j.1558-5646.1978.tb04589.x
- ↑ Diane M. B. Dodd and Jeffrey R. Powell (1985), "Founder-Flush Speciation: An Update of Experimental Results with Drosophila", Evolution, 39 (6): 1388–1392, PMID 28564258, doi:10.1111/j.1558-5646.1985.tb05704.x
- ↑ Lorna H. Arita and Kenneth Y. Kaneshiro (1979), "Ethological Isolation Between Two Stocks of Drosophila Adiastola Hardy", Proc. Hawaii. Entomol. Soc., 13: 31–34
- ↑ J. N. Ahearn (1980), "Evolution of behavioral reproductive isolation in a laboratory stock of Drosophila silvestris", Experientia, 36 (1): 63–64, doi:10.1007/BF02003975
- ↑ Diane M. B. Dodd and Jeffrey R. Powell (1985), "Founder-Flush Speciation: An Update of Experimental Results with Drosophila", Evolution, 39 (6): 1388–1392, PMID 28564258, doi:10.1111/j.1558-5646.1985.tb05704.x
- ↑ John Ringo, David Wood, Robert Rockwell, and Harold Dowse (1985), "An Experiment Testing Two Hypotheses of Speciation", The American Naturalist, 126 (5): 642–661
- ↑ Meffert, L. M. and Bryant, E. H. (1991), "Mating propensity and courtship behavior in serially bottlenecked lines of the housefly", Evolution, 45 (2): 293–306, PMID 28567864, doi:10.1111/j.1558-5646.1991.tb04404.x
- ↑ Galiana, A., Moya, A. and Ayala, F. J. (1993), "Founder-flush speciation in Drosophila pseudoobscura: a large scale experiment", Evolution, 47 (2): 432–444, PMID 28568735, doi:10.1111/j.1558-5646.1993.tb02104.x
- ↑ Rundle, H. D., Mooers, A. Ø. and Whitlock, M. C. (1998), "Single founder-flush events and the evolution of reproductive isolation", Evolution, 52 (6): 1850–1855, PMID 28565304, doi:10.1111/j.1558-5646.1998.tb02263.x
- ↑ Mooers, A. Ø., Rundle, H. D. and Whitlock, M. C. (1999), "The effects of selection and bottlenecks on male mating success in peripheral isolates", American Naturalist, 153: 437–444