Pentadienyl
In chemistry, pentadienyl refers to the organic radical, anion, or cation with the formula [[CH<sub>2</sub>CHCCHCHCH<sub>2</sub>]]z, where z = 0, -, +, respectively.
Organometallic chemistry
In organometallic chemistry, the pentadienyl anion is a ligand, the acyclic analogue of the more common cyclopentadienyl. It is generated by deprotonation of pentadiene. A number of complexes are known, including the analogue of ferrocene, [[Fe(C<sub>5</sub>H<sub>7</sub>)<sub>2</sub>]]. Only few pentadienyl complexes feature C5H7 ligands. More common is the dimethyl analogue 2,4-Me2C5H5. Additionally, many pentadienyl ligands are cyclic, being derived from the addition of hydride to η6-arene complexes or hydride abstraction from cyclohexadiene complexes.[1][2]
The first pentadienyl complex to be reported was derived from protonolysis of a complex of pentadienol:[3]
- Fe(C5H7OH)(CO)3 + H+ → [Fe(C5H7)(CO)3]+ + H2O
Treatment of this cation with sodium borohydride gives the pentadiene complex:
- [Fe(C5H7)(CO)3]+ + H− → Fe(C5H8)(CO)3
Organic chemistry
In organic chemistry, the pentadienyl radical is of some significance as an especially stabilized radical. The radical is delocalized over five carbon centers. Consequently the C-H bond in the diene (CH2(CH=CH2)) is especially weak. Fats derivatives containing this "doubly allylic" group are collectively called drying oils. They tend to polymerize in a useful way upon exposure to air.
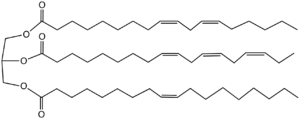 Representative triglyceride found in a drying oil. The two upper fatty esters display high reactivity toward air owing to their tendency to form pentadienyl and heptadienyl radicals, respectively.
Representative triglyceride found in a drying oil. The two upper fatty esters display high reactivity toward air owing to their tendency to form pentadienyl and heptadienyl radicals, respectively.
Biochemistry
Cyclooxygenases ("COX") are an enzymes that generate prostanoids, including thromboxane and prostaglandins such as prostacyclin. Aspirin and ibuprofen exert their effects through inhibition of COX.

References
- ↑ Lothar Stahl, Richard D. Ernst (2007). "Pentadienyl Complexes of the Group 4 Transition Metals". Advances in Organometallic Chemistry. 55: 137–199. doi:10.1016/S0065-3055(07)55003-3.
- ↑ Richard D. Ernst (1988). "Structural and reactivity patterns in transition-metal-pentadienyl chemistry". Chem. Rev. 88: =1255–1291. doi:10.1021/cr00089a013.
- ↑ Mahler, J. E.; Pettit, R. (1962). "Pentadienyl and Hexadienyl Carbonium Ions as Ligands in Stable Complex Cations". J. Am. Chem. Soc. 84: 1511–2. doi:10.1021/ja00867a051.