Parkinson's disease
| Parkinson's disease | |
|---|---|
| Synonyms | Parkinson disease, idiopathic or primary parkinsonism, hypokinetic rigid syndrome, paralysis agitans |
.png) | |
| Illustration of Parkinson's disease by William Richard Gowers, first published in A Manual of Diseases of the Nervous System (1886) | |
| Specialty | Neurology |
| Symptoms | Shaking, rigidity, slowness of movement, difficulty walking[1] |
| Complications | Dementia, depression, anxiety[2] |
| Usual onset | Age over 60[1][3] |
| Causes | Unknown[4] |
| Risk factors | Pesticide exposure, head injuries[4] |
| Diagnostic method | Based on symptoms[1] |
| Similar conditions | Dementia with Lewy bodies, progressive supranuclear palsy, essential tremor, antipsychotic use[5] |
| Treatment | Medications, surgery[1] |
| Medication | L-DOPA, dopamine agonists[2] |
| Prognosis | Life expectancy ~ 10 years[2] |
| Frequency | 6.2 million (2015)[6] |
| Deaths | 117,400 (2015)[7] |
Parkinson's disease (PD) is a long-term degenerative disorder of the central nervous system that mainly affects the motor system.[1] The symptoms generally come on slowly over time.[1] Early in the disease, the most obvious are shaking, rigidity, slowness of movement, and difficulty with walking.[1] Thinking and behavioral problems may also occur.[2] Dementia becomes common in the advanced stages of the disease.[2] Depression and anxiety are also common occurring in more than a third of people with PD.[2] Other symptoms include sensory, sleep, and emotional problems.[1][2] The main motor symptoms are collectively called "parkinsonism", or a "parkinsonian syndrome".[4][8]
The cause of Parkinson's disease is generally unknown, but believed to involve both genetic and environmental factors.[4] Those with a family member affected are more likely to get the disease themselves.[4] There is also an increased risk in people exposed to certain pesticides and among those who have had prior head injuries, while there is a reduced risk in tobacco smokers and those who drink coffee or tea.[4][9] The motor symptoms of the disease result from the death of cells in the substantia nigra, a region of the midbrain.[1] This results in not enough dopamine in these areas.[1] The reason for this cell death is poorly understood, but involves the build-up of proteins into Lewy bodies in the neurons.[4] Diagnosis of typical cases is mainly based on symptoms, with tests such as neuroimaging being used to rule out other diseases.[1]
There is no cure for Parkinson's disease.[1] Initial treatment is typically with the antiparkinson medication levodopa (L-DOPA), with dopamine agonists being used once levodopa becomes less effective.[2] As the disease progresses and neurons continue to be lost, these medications become less effective while at the same time they produce a complication marked by involuntary writhing movements.[2] Diet and some forms of rehabilitation have shown some effectiveness at improving symptoms.[10][11] Surgery to place microelectrodes for deep brain stimulation has been used to reduce motor symptoms in severe cases where drugs are ineffective.[1] Evidence for treatments for the non-movement-related symptoms of PD, such as sleep disturbances and emotional problems, is less strong.[4]
In 2015, PD affected 6.2 million people and resulted in about 117,400 deaths globally.[6][7] Parkinson's disease typically occurs in people over the age of 60, of which about one percent are affected.[1][3] Males are more often affected than females.[4] When it is seen in people before the age of 50, it is called young-onset PD.[12] The average life expectancy following diagnosis is between 7 and 14 years.[2] The disease is named after the English doctor James Parkinson, who published the first detailed description in An Essay on the Shaking Palsy, in 1817.[13][14] Public awareness campaigns include World Parkinson's Day (on the birthday of James Parkinson, 11 April) and the use of a red tulip as the symbol of the disease.[15] People with parkinsonism who have increased the public's awareness of the condition include actor Michael J. Fox, Olympic cyclist Davis Phinney, and late professional boxer Muhammad Ali.[16][17][18]
Classification
The movement difficulties found in PD are called "parkinsonism" and a number of different disorders feature parkinsonism. "Parkinsonism" is defined as bradykinesia (slowness in initiating voluntary movements, with progressive reduction in speed and range of repetitive actions such as voluntary finger-tapping[19]) in combination with one of three other physical signs: muscular (lead-pipe or cogwheel) rigidity, tremor at rest, and postural instability.[20][21]
Parkinson's disease is the most common form of parkinsonism and is sometimes called "idiopathic parkinsonism", meaning parkinsonism with no identifiable cause.[22][23] Identifiable causes of parkinsonism include toxins, infections, side effects of drugs, metabolic derangement, and brain lesions such as strokes. Several neurodegenerative disorders also may present with parkinsonism and are sometimes referred to as "atypical parkinsonism" or "Parkinson plus" syndromes (illnesses with parkinsonism plus some other features distinguishing them from PD). They include multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration, and dementia with Lewy bodies (DLB).[22][24]
Scientists sometimes refer to Parkinson’s disease as a synucleiopathy (due to an abnormal accumulation of alpha-synuclein protein in the brain) to distinguish it from other neurodegenerative diseases, such as Alzheimer's disease where the brain accumulates tau protein.[25] Considerable clinical and pathological overlap exists between tauopathies and synucleinopathies. In contrast to Parkinson's disease, Alzheimer's disease presents most commonly with memory loss, and the cardinal signs of Parkinson's disease (slowness, tremor, stiffness, and postural instability) are not normal features of Alzheimer's.
Dementia with Lewy bodies is another synucleinopathy and it has close pathological similarities with PD, especially with the subset of PD cases with dementia. The relationship between PD and DLB is complex and incompletely understood.[26] They may represent parts of a continuum with variable distinguishing clinical and pathological features or they may prove to be separate diseases.[26]
Signs and symptoms

The most recognizable symptoms in Parkinson's disease are movement ("motor") related.[29] Non-motor symptoms, which include autonomic dysfunction, neuropsychiatric problems (mood, cognition, behavior or thought alterations), and sensory (especially altered sense of smell) and sleep difficulties, are also common. Some of these non-motor symptoms may be present at the time of diagnosis.[29]
Motor
Four motor symptoms are considered cardinal in PD: tremor, slowness of movement (bradykinesia), rigidity, and postural instability.[29]
The most common presenting sign is a coarse slow tremor of the hand at rest which disappears during voluntary movement of the affected arm and in the deeper stages of sleep.[29] It typically appears in only one hand, eventually affecting both hands as the disease progresses.[29] Frequency of PD tremor is between 4 and 6 hertz (cycles per second). A feature of tremor is pill-rolling, the tendency of the index finger and thumb to touch and perform together a circular movement.[29][30] The term derives from the similarity between the movement of people with PD and the early pharmaceutical technique of manually making pills.[30]
Bradykinesia (slowness of movement) is found in every case of PD, and is due to disturbances in motor planning of movement initiation, and associated with difficulties along the whole course of the movement process, from planning to initiation to execution of a movement. Performance of sequential and simultaneous movement is impaired. Bradykinesia is the most handicapping symptom of Parkinson’s disease leading to difficulties with everyday tasks such as dressing, feeding, and bathing. It leads to particular difficulty in carrying out two independent motor activities at the same time and can be made worse by emotional stress or intercurrent illnesses. Paradoxically patients with Parkinson's disease can often ride a bicycle or climb stairs more easily than walk on a level. While most physicians may readily notice bradykinesia, formal assessment requires a patient to do repetitive movements with their fingers and feet.[31]
Rigidity is stiffness and resistance to limb movement caused by increased muscle tone, an excessive and continuous contraction of muscles.[29] In parkinsonism the rigidity can be uniform ("lead-pipe rigidity") or ratchety ("cogwheel rigidity").[22][29][32][33] The combination of tremor and increased tone is considered to be at the origin of cogwheel rigidity.[34] Rigidity may be associated with joint pain; such pain being a frequent initial manifestation of the disease.[29] In early stages of Parkinson's disease, rigidity is often asymmetrical and it tends to affect the neck and shoulder muscles prior to the muscles of the face and extremities.[35] With the progression of the disease, rigidity typically affects the whole body and reduces the ability to move.
Postural instability is typical in the later stages of the disease, leading to impaired balance and frequent falls,[36] and secondarily to bone fractures, loss of confidence, and reduced mobility.[37] Instability is often absent in the initial stages, especially in younger people, especially prior to the development of bilateral symptoms.[38] Up to 40% of people diagnosed with PD may experience falls and around 10% may have falls weekly, with the number of falls being related to the severity of PD.[29]
Other recognized motor signs and symptoms include gait and posture disturbances such as festination (rapid shuffling steps and a forward-flexed posture when walking with no flexed arm swing). Freezing of gait (brief arrests when the feet seem to get stuck to the floor, especially on turning or changing direction), a slurred monotonous quiet voice, mask-like facial expression, and handwriting that gets smaller and smaller are other common signs.[39]
Neuropsychiatric
Parkinson's disease can cause neuropsychiatric disturbances, which can range from mild to severe. This includes disorders of cognition, mood, behavior, and thought.[29]
Cognitive disturbances can occur in the early stages of the disease and sometimes prior to diagnosis, and increase in prevalence with duration of the disease.[29][40] The most common cognitive deficit in PD is executive dysfunction, which can include problems with planning, cognitive flexibility, abstract thinking, rule acquisition, inhibiting inappropriate actions, initiating appropriate actions, working memory, and control of attention.[40][41] Other cognitive difficulties include slowed cognitive processing speed, impaired recall and impaired perception and estimation of time.[40][41] Nevertheless, improvement appears when recall is aided by cues.[40] Visuospatial difficulties are also part of the disease, seen for example when the individual is asked to perform tests of facial recognition and perception of the orientation of drawn lines.[40][41] A person with PD has two to six times the risk of dementia compared to the general population.[29][40]
The prevalence of dementia increases with age and, to a lesser degree, duration of the disease.[42] Dementia is associated with a reduced quality of life in people with PD and their caregivers, increased mortality, and a higher probability of needing nursing home care.[40]
Impulse control disorders including pathological gambling, compulsive sexual behavior, binge eating, compulsive shopping and reckless generosity can be caused by medication, particularly orally active dopamine agonists. The dopamine dysregulation syndrome - with wanting of medication leading to overusage - is a rare complication of levodopa use (Giovannoni, et al. 2000).
Behavior and mood alterations are more common in PD without cognitive impairment than in the general population, and are usually present in PD with dementia. The most frequent mood difficulties are depression, apathy, and anxiety.[29] Establishing the diagnosis of depression is complicated by the fact that the body language of depression may masquerade as PD including a sad expressionless anxious face, a hang dog appearance, slow movement, and monotonous speech. Up to 30% of people with PD may experience symptoms of anxiety, ranging from a generalized anxiety disorder to social phobia, panic disorders and obsessive compulsive disorders. They contribute to impaired quality of life and increased severity of motor symptoms such as on/off fluctuations or freezing episodes.
Punding in which complicated repetitive aimless stereotyped behaviors occur for many hours is another disturbance caused by anti-Parkinson medication.
Hallucinations or delusions occur in approximately 50% of people with PD over the course of the illness, and may herald the emergence of dementia. These range from minor hallucinations - "sense of passage" (something quickly passing beside the person) or "sense of presence" (the perception of something/someone standing just to the side or behind the person) - to full blown vivid, formed visual hallucinations and paranoid ideation. Auditory hallucinations are uncommon in PD, and are rarely described as voices. It is now believed that psychosis is an integral part of the disease. A psychosis with delusions and associated delirium is a recognized complication of anti-Parkinson drug treatment and may also be caused by urinary tract infections (as frequently occurs in the fragile elderly), but drugs and infection are not the only factors, and underlying brain pathology or changes in neurotransmitters or their receptors (e.g., acetylcholine, serotonin) are also thought to play a role in psychosis in PD.[43][44]
Other
In addition to neuropsychiatric and motor symptoms, PD can impair other functions.
Sleep problems are a feature of the disease and can be worsened by medications.[29] Symptoms can manifest as daytime drowsiness (including sudden sleep attacks resembling narcolepsy), disturbances in REM sleep, or insomnia.[29] REM behavior disorder (RBD), in which patients act out dreams, sometimes injuring themselves or their bed partner, may begin many years before the development of motor or cognitive features of PD or DLB.[45]
Alterations in the autonomic nervous system can lead to orthostatic hypotension (low blood pressure upon standing), oily skin and excessive sweating, urinary incontinence, and altered sexual function.[29] Constipation and impaired stomach emptying (gastric dysmotility) can be severe enough to cause discomfort and even endanger health.[10] Changes in perception may include an impaired sense of smell, disturbed vision, pain, and paresthesia (tingling and numbness).[29] All of these symptoms can occur years before diagnosis of the disease.[29]
Causes
Environmental factors
Exposure to pesticides and a history of head injury have each been linked with Parkinson disease (PD), but the risks are modest. Never having smoked cigarettes, and never drinking caffeinated beverages, are also associated with small increases in risk of developing PD.[46]
Low concentrations of urate in the blood serum is associated with an increased risk of PD.[47]
Genetics

Research indicates that PD is the product of a complex interaction of genetic and environmental factors.[4] Around 15% of individuals with PD have a first-degree relative who has the disease,[22] and 5–10% of people with PD are known to have other forms of the disease that occur because of a mutation in one of several specific genes.[48] Harboring one of these gene mutations may not lead to the disease; susceptibility factors put the individual at an increased risk, often in combination with other risk factors, which also affect age of onset, severity and progression.[48]
Genes implicated in the development of PD include SNCA, LRRK2, GBA, PRKN, PINK1, PARK7, VPS35, EIF4G1, DNAJC13 and CHCHD2.[49]
SNCA gene mutations are important in PD because the protein that gene encodes, alpha-synuclein, is the main component of the Lewy bodies that accumulate in the brains of people with PD.[48] Mutations in some genes, including SNCA, LRRK2 and GBA, have been found to be risk factors for "sporadic" (non-familial) PD.[48] Mutations in the gene LRRK2 are the most common known cause of familial and sporadic PD, accounting for approximately 5% of individuals with a family history of the disease and 3% of sporadic cases.[50][48] A mutation in GBA presents the greatest genetic risk of developing Parkinsons disease.[49]
Several Parkinson-related genes are involved in the function of lysosomes, organelles that digest cellular waste products. It has been suggested that some cases of PD may be caused by lysosome dysfunctions that reduce the ability of cells to break down alpha-synuclein.[51]
Pathophysiology
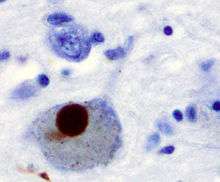
The main pathological characteristics of PD are cell death in the brain's basal ganglia (affecting up to 70% of the dopamine secreting neurons in the substantia nigra pars compacta by the end of life)[50] and the presence of Lewy bodies (accumulations of the protein alpha-synuclein) in many of the remaining neurons. This loss of neurons is accompanied by the death of astrocytes (star-shaped glial cells) and a significant increase in the number of microglia (another type of glial cell) in the substantia nigra.[52]
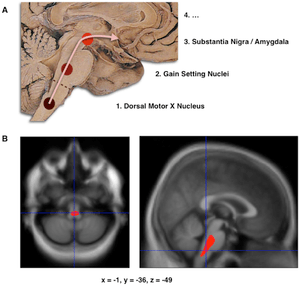
- Schematic initial progression of Lewy body deposits in the first stages of Parkinson's disease, as proposed by Braak and colleagues
- Localization of the area of significant brain volume reduction in initial PD compared with a group of participants without the disease in a neuroimaging study, which concluded that brainstem damage may be the first identifiable stage of PD neuropathology[53]
There are five major pathways in the brain connecting other brain areas with the basal ganglia. These are known as the motor, oculo-motor, associative, limbic and orbitofrontal circuits, with names indicating the main projection area of each circuit.[54] All of them are affected in PD, and their disruption explains many of the symptoms of the disease, since these circuits are involved in a wide variety of functions, including movement, attention and learning.[54] Scientifically, the motor circuit has been examined the most intensively.[54]
A particular conceptual model of the motor circuit and its alteration with PD has been of great influence since 1980, although some limitations have been pointed out which have led to modifications.[54] In this model, the basal ganglia normally exert a constant inhibitory influence on a wide range of motor systems, preventing them from becoming active at inappropriate times. When a decision is made to perform a particular action, inhibition is reduced for the required motor system, thereby releasing it for activation. Dopamine acts to facilitate this release of inhibition, so high levels of dopamine function tend to promote motor activity, while low levels of dopamine function, such as occur in PD, demand greater exertions of effort for any given movement. Thus, the net effect of dopamine depletion is to produce hypokinesia, an overall reduction in motor output.[54] Drugs that are used to treat PD, conversely, may produce excessive dopamine activity, allowing motor systems to be activated at inappropriate times and thereby producing dyskinesias.[54]
Brain cell death
There is speculation of several mechanisms by which the brain cells could be lost.[55] One mechanism consists of an abnormal accumulation of the protein alpha-synuclein bound to ubiquitin in the damaged cells. This insoluble protein accumulates inside neurones forming inclusions called Lewy bodies.[50][56] According to the Braak staging, a classification of the disease based on pathological findings, Lewy bodies first appear in the olfactory bulb, medulla oblongata and pontine tegmentum; individuals at this stage may be asymptomatic or may have early non-motor symptoms (such as loss of sense of smell, or some sleep or automatic dysfunction). As the disease progresses, Lewy bodies develop in the substantia nigra, areas of the midbrain and basal forebrain and, finally, the neocortex.[50] These brain sites are the main places of neuronal degeneration in PD; however, Lewy bodies may not cause cell death and they may be protective (with the abnormal protein sequestered or walled off). Other forms of alpha-synuclein (e.g., oligomers) that are not aggregated in Lewy bodies and Lewy neurites may actually be the toxic forms of the protein.[55][56] In people with dementia, a generalized presence of Lewy bodies is common in cortical areas. Neurofibrillary tangles and senile plaques, characteristic of Alzheimer's disease, are not common unless the person is demented.[52]
Other cell-death mechanisms include proteasomal and lysosomal system dysfunction and reduced mitochondrial activity.[55] Iron accumulation in the substantia nigra is typically observed in conjunction with the protein inclusions. It may be related to oxidative stress, protein aggregation and neuronal death, but the mechanisms are not fully understood.[57]
Diagnosis
A physician will initially assess for Parkinson's disease with a careful medical history and neurological examination.[29] People may be given levodopa, with any resulting improvement in motor impairment helping to confirm the PD diagnosis. The finding of Lewy bodies in the midbrain on autopsy is usually considered final proof that the person had PD. The clinical course of the illness over time may reveal it is not Parkinson's disease, requiring that the clinical presentation be periodically reviewed to confirm accuracy of the diagnosis.[29][58]
Other causes that can secondarily produce parkinsonism are stroke and drugs.[58] Parkinson plus syndromes such as progressive supranuclear palsy and multiple system atrophy must be ruled out.[29] Anti-Parkinson's medications are typically less effective at controlling symptoms in Parkinson plus syndromes.[29] Faster progression rates, early cognitive dysfunction or postural instability, minimal tremor or symmetry at onset may indicate a Parkinson plus disease rather than PD itself.[59] Genetic forms with an autosomal dominant or recessive pattern of inheritance are sometimes referred to as familial Parkinson's disease or familial parkinsonism.[22]
Medical organizations have created diagnostic criteria to ease and standardize the diagnostic process, especially in the early stages of the disease. The most widely known criteria come from the UK Queen Square Brain Bank for Neurological Disorders and the U.S. National Institute of Neurological Disorders and Stroke. The Queen Square Brain Bank criteria require slowness of movement (bradykinesia) plus either rigidity, resting tremor, or postural instability. Other possible causes of these symptoms need to be ruled out. Finally, three or more of the following supportive features are required during onset or evolution: unilateral onset, tremor at rest, progression in time, asymmetry of motor symptoms, response to levodopa for at least five years, clinical course of at least ten years and appearance of dyskinesias induced by the intake of excessive levodopa.[60]
When PD diagnoses are checked by autopsy, movement disorders experts are found on average to be 79.6% accurate at initial assessment and 83.9% accurate after they have refined their diagnosis at a follow-up examination. When clinical diagnoses performed mainly by nonexperts are checked by autopsy, average accuracy is 73.8%. Overall, 80.6% of PD diagnoses are accurate, and 82.7% of diagnoses using the Brain Bank criteria are accurate.[61]
A task force of the International Parkinson and Movement Disorder Society (MDS) has proposed diagnostic criteria for Parkinson’s disease as well as research criteria for the diagnosis of prodromal disease, but these will require validation against the more established criteria.[62][63]
Imaging
Computed tomography (CT) scans of people with PD usually appear normal.[64] MRI has become more accurate in diagnosis of the disease over time, specifically through iron-sensitive T2* and SWI sequences at a magnetic field strength of at least 3T, both of which can demonstrate absence of the characteristic 'swallow tail' imaging pattern in the dorsolateral substantia nigra.[65] In a meta-analysis, absence of this pattern was 98% sensitive and 95% specific for the disease.[66] Diffusion MRI has shown potential in distinguishing between PD and Parkinson plus syndromes, though its diagnostic value is still under investigation.[64] CT and MRI are also used to rule out other diseases that can be secondary causes of parkinsonism, most commonly encephalitis and chronic ischemic insults, as well as less frequent entities such as basal ganglia tumors and hydrocephalus.[64]
Dopamine-related activity in the basal ganglia can be directly measured with PET and SPECT scans. A finding of reduced dopamine-related activity in the basal ganglia can rule out drug-induced parkinsonism, but reduced basal ganglia dopamine-related activity is seen in both PD and the Parkinson-plus disorders so these scans are not reliable in distinguishing PD from other neurodegenerative causes of parkinsonism.[64]
Prevention
Exercise in middle age may reduce the risk of Parkinson's disease later in life.[11] Caffeine also appears protective with a greater decrease in risk occurring with a larger intake of caffeinated beverages such as coffee.[67] People who smoke cigarettes or use smokeless tobacco are less likely than non-smokers to develop PD, and the more they have used tobacco, the less likely they are to develop PD. It is not known what underlies this effect. Tobacco use may actually protect against PD, or it may be that an unknown factor both increases the risk of PD and causes an aversion to tobacco or makes it easier to quit using tobacco.[68]
Antioxidants, such as vitamins C and E, have been proposed to protect against the disease, but results of studies have been contradictory and no positive effect has been proven.[69] The results regarding fat and fatty acids have been contradictory, with various studies reporting protective effects, risk-increasing effects or no effects.[69] There have been preliminary indications that the use of anti-inflammatory drugs and calcium channel blockers may be protective.[4] A 2010 meta-analysis found that nonsteroidal anti-inflammatory drugs (apart from aspirin), have been associated with at least a 15 percent (higher in long-term and regular users) reduction of incidence of the development of Parkinson's disease.[70]
Management

There is no cure for Parkinson's disease, but medications, surgery, and physical treatment can provide relief and are much more effective than treatments available for other neurological disorders like Alzheimer’s disease, motor neurone disease, the Parkinson plus syndromes and multiple sclerosis. The main families of drugs useful for treating motor symptoms are levodopa (always combined with a dopa decarboxylase inhibitor and sometimes also with a COMT inhibitor), dopamine agonists and MAO-B inhibitors. The stage of the disease and the age at disease onset determine which group is most useful.[71]
Three stages may be distinguished: an initial stage in which the individual with PD has already developed some disability requiring pharmacological treatment, a second stage associated with the development of complications related to levodopa usage, and a third stage when symptoms unrelated to dopamine deficiency or levodopa treatment may predominate.[72]
Treatment in the first stage aims for an optimal trade off between symptom control and treatment side-effects. The start of levodopa treatment may be postponed by initially using other medications such as MAO-B inhibitors and dopamine agonists instead, in the hope of delaying the onset of complications due to levodopa use.[73] However, levodopa is still the most effective treatment for the motor symptoms of PD and should not be delayed in patients whose quality of life is impaired by those symptoms. Levodopa-related dyskinesias correlate more strongly with duration and severity of the disease than duration of levodopa treatment, so delaying this therapy may not really provide much longer dyskinesia-free time than early use.[74]
In the second stage the aim is to reduce PD symptoms while controlling fluctuations in the effect of the medication. Sudden withdrawals from medication or overuse have to be managed.[73] When oral medications are not enough to control symptoms, surgery, deep brain stimulation, subcutaneous waking day apomorphine infusion and enteral dopa pumps can be of use.[75] The third stage presents many challenging problems requiring a variety of treatments for psychiatric symptoms, orthostatic hypotension, bladder dysfunction, etc.[75] In the final stages of the disease, palliative care is provided to improve quality of life.[76]
Medications
Levodopa
The motor symptoms of PD are the result of reduced dopamine production in the brain's basal ganglia. Dopamine does not cross the blood-brain barrier, so it cannot be taken as a medicine to boost the brain's depleted levels of dopamine. However a precursor of dopamine, levodopa, can pass through to the brain where it is readily converted to dopamine, and administration of levodopa temporarily diminishes the motor symptoms of PD. Levodopa has been the most widely used PD treatment for over 40 years.[73]
Only 5–10% of levodopa crosses the blood–brain barrier. Much of the remainder is metabolized to dopamine elsewhere in the body, causing a variety of side effects including nausea, vomiting and orthostatic hypotension.[77] Carbidopa and benserazide are dopa decarboxylase inhibitors which do not cross the blood-brain barrier and inhibit the conversion of levodopa to dopamine outside the brain, reducing side effects and improving the availability of levodopa for passage into the brain. One of these drugs is usually taken along with levodopa, often combined with levodopa in the same pill.[78]
Levodopa use leads in the long term to the development of complications: involuntary movements called dyskinesias, and fluctuations in the effectiveness of the medication.[73] When fluctuations occur, a person can cycle through phases with good response to medication and reduced PD symptoms ("on" state), and phases with poor response to medication and significant PD symptoms ("off" state).[73] Using lower doses of levodopa may reduce the risk and severity of these levodopa-induced complications.[79] A former strategy to reduce levodopa-related dyskinesia and fluctuations was to withdraw levodopa medication for some time. This is now discouraged since it can bring on dangerous side effects such as neuroleptic malignant syndrome.[73] Most people with PD will eventually need levodopa and will later develop levodopa-induced fluctuations and dyskinesias.[73]
There are controlled-release versions of levodopa. Older controlled-release levodopa preparations have poor and unreliable absorption and bioavailability and have not demonstrated improved control of PD motor symptoms or a reduction in levodopa-related complications when compared to immediate release preparations. A newer extended-release levodopa preparation does seem to be more effective in reducing fluctuations but in many patients problems persist. Intestinal infusions of levodopa (Duodopa) can result in striking improvements in fluctuations compared to oral levodopa when the fluctuations are due to insufficient uptake caused by gastroparesis. Other oral, longer acting formulations are under study and other modes of delivery (inhaled, transdermal) are being developed.[78]
COMT inhibitors
Tolcapone inhibits the activity COMT, an enzyme which degrades dopamine.[73] It has been used to complement levodopa; however, its usefulness is limited by possible complications such as liver damage.[73] A similarly effective drug, entacapone, has not been shown to cause significant alterations of liver function.[73] Licensed preparations of entacapone contain entacapone alone or in combination with carbidopa and levodopa.[73]
Dopamine agonists
Several dopamine agonists that bind to dopamine receptors in the brain have similar effects to levodopa.[73] These were initially used as a complementary therapy to levodopa for individuals experiencing levodopa complications (on-off fluctuations and dyskinesias); they are now mainly used on their own as first therapy for the motor symptoms of PD with the aim of delaying the initiation of levodopa therapy and so delaying the onset of levodopa's complications.[73][80] Dopamine agonists include bromocriptine, pergolide, pramipexole, ropinirole, piribedil, cabergoline, apomorphine and lisuride.
Though dopamine agonists are less effective than levodopa at controlling PD motor symptoms, they are usually effective enough to manage these symptoms in the first years of treatment.[22] Dyskinesias due to dopamine agonists are rare in younger people who have PD but, along with other complications, become more common with older age at onset.[22] Thus dopamine agonists are the preferred initial treatment for younger onset PD, and levodopa is preferred for older onset PD.[22]
Dopamine agonists produce significant, although usually mild, side effects including drowsiness, hallucinations, insomnia, nausea, and constipation.[73] Sometimes side effects appear even at a minimal clinically effective dose, leading the physician to search for a different drug.[73] Agonists have been related to impulse control disorders (such as compulsive sexual activity, eating, gambling and shopping) even more strongly than levodopa.[81] They tend to be more expensive than levodopa.[22]
Apomorphine, a non-orally administered dopamine agonist, may be used to reduce off periods and dyskinesia in late PD.[73] It is administered by intermittent injections or continuous subcutaneous infusions.[73] Since secondary effects such as confusion and hallucinations are common, individuals receiving apomorphine treatment should be closely monitored.[73] Two dopamine agonists that are administered through skin patches (lisuride and rotigotine) and are useful for people in the initial stages and possibly to control off states in those in the advanced state.[82]
MAO-B inhibitors
MAO-B inhibitors (safinamide, selegiline and rasagiline) increase the amount of dopamine in the basal ganglia by inhibiting the activity of monoamine oxidase B (MAO-B), an enzyme which breaks down dopamine.[73] Like dopamine agonists, their use may delay the commencement of levodopa therapy in early disease, but MAO-B inhibitors produce more adverse effects and are less effective than levodopa at controlling PD motor symptoms. There are few studies of their effectiveness in the advanced stage, although results suggest that they are useful to reduce fluctuations between on and off periods.[73] An initial study indicated that selegiline in combination with levodopa increased the risk of death, but this was later disproven.[73]
Other drugs
Other drugs such as amantadine and anticholinergics may be useful as treatment of motor symptoms. However, the evidence supporting them lacks quality, so they are not first choice treatments.[73] In addition to motor symptoms, PD is accompanied by a diverse range of symptoms. A number of drugs have been used to treat some of these problems.[83] Examples are the use of quetiapine for psychosis, cholinesterase inhibitors for dementia, and modafinil for daytime sleepiness.[83][84]
Surgery

Treating motor symptoms with surgery was once a common practice, but since the discovery of levodopa, the number of operations declined.[85] Studies in the past few decades have led to great improvements in surgical techniques, so that surgery is again being used in people with advanced PD for whom drug therapy is no longer sufficient.[85] Surgery for PD can be divided in two main groups: lesional and deep brain stimulation (DBS). Target areas for DBS or lesions include the thalamus, the globus pallidus or the subthalamic nucleus.[85] Deep brain stimulation is the most commonly used surgical treatment, developed in the 1980s by Alim Louis Benabid and others. It involves the implantation of a medical device called a neurostimulator, which sends electrical impulses to specific parts of the brain. DBS is recommended for people who have PD with motor fluctuations and tremor inadequately controlled by medication, or to those who are intolerant to medication, as long as they do not have severe neuropsychiatric problems.[86] Other, less common, surgical therapies involve intentional formation of lesions to suppress overactivity of specific subcortical areas. For example, pallidotomy involves surgical destruction of the globus pallidus to control dyskinesia.[85]
Rehabilitation
Exercise programs are recommended in people with Parkinson's disease.[11] There is some evidence that speech or mobility problems can improve with rehabilitation, although studies are scarce and of low quality.[87][88] Regular physical exercise with or without physical therapy can be beneficial to maintain and improve mobility, flexibility, strength, gait speed, and quality of life.[88] When an exercise program is performed under the supervision of a physiotherapist, there are more improvements in motor symptoms, mental and emotional functions, daily living activities, and quality of life compared to a self-supervised exercise program at home.[89] In terms of improving flexibility and range of motion for people experiencing rigidity, generalized relaxation techniques such as gentle rocking have been found to decrease excessive muscle tension. Other effective techniques to promote relaxation include slow rotational movements of the extremities and trunk, rhythmic initiation, diaphragmatic breathing, and meditation techniques.[90] As for gait and addressing the challenges associated with the disease such as hypokinesia (slowness of movement), shuffling and decreased arm swing; physiotherapists have a variety of strategies to improve functional mobility and safety. Areas of interest with respect to gait during rehabilitation programs focus on, but are not limited to improving gait speed, the base of support, stride length, trunk and arm swing movement. Strategies include utilizing assistive equipment (pole walking and treadmill walking), verbal cueing (manual, visual and auditory), exercises (marching and PNF patterns) and altering environments (surfaces, inputs, open vs. closed).[91] Strengthening exercises have shown improvements in strength and motor function for people with primary muscular weakness and weakness related to inactivity with mild to moderate Parkinson's disease. However, reports show a significant interaction between strength and the time the medications was taken. Therefore, it is recommended that people with PD should perform exercises 45 minutes to one hour after medications when they are at their best.[92] Also, due to the forward flexed posture, and respiratory dysfunctions in advanced Parkinson's disease, deep diaphragmatic breathing exercises are beneficial in improving chest wall mobility and vital capacity.[93] Exercise may improve constipation.[10]
One of the most widely practiced treatments for speech disorders associated with Parkinson's disease is the Lee Silverman voice treatment (LSVT).[87][94] Speech therapy and specifically LSVT may improve speech.[87] Occupational therapy (OT) aims to promote health and quality of life by helping people with the disease to participate in as many of their daily living activities as possible.[87] There have been few studies on the effectiveness of OT and their quality is poor, although there is some indication that it may improve motor skills and quality of life for the duration of the therapy.[87][95]
Palliative care
Palliative care is specialized medical care for people with serious illnesses, including Parkinson's. The goal of this speciality is to improve quality of life for both the person suffering from Parkinson's and the family by providing relief from the symptoms, pain, and stress of illnesses.[96] As Parkinson's is not a curable disease, all treatments are focused on slowing decline and improving quality of life, and are therefore palliative in nature.[97]
Palliative care should be involved earlier, rather than later in the disease course.[98][99] Palliative care specialists can help with physical symptoms, emotional factors such as loss of function and jobs, depression, fear, and existential concerns.[98][99][100]
Along with offering emotional support to both the patient and family, palliative care serves an important role in addressing goals of care. People with Parkinson's may have many difficult decisions to make as the disease progresses such as wishes for feeding tube, non-invasive ventilator, and tracheostomy; wishes for or against cardiopulmonary resuscitation; and when to use hospice care.[97] Palliative care team members can help answer questions and guide people with Parkinson's on these complex and emotional topics to help them make the best decision based on their own values.[99][101]
Other treatments
Muscles and nerves that control the digestive process may be affected by PD, resulting in constipation and gastroparesis (food remaining in the stomach for a longer period than normal).[10] A balanced diet, based on periodical nutritional assessments, is recommended and should be designed to avoid weight loss or gain and minimize consequences of gastrointestinal dysfunction.[10] As the disease advances, swallowing difficulties (dysphagia) may appear. In such cases it may be helpful to use thickening agents for liquid intake and an upright posture when eating, both measures reducing the risk of choking. Gastrostomy to deliver food directly into the stomach is possible in severe cases.[10]
Levodopa and proteins use the same transportation system in the intestine and the blood–brain barrier, thereby competing for access.[10] When they are taken together, this results in a reduced effectiveness of the drug.[10] Therefore, when levodopa is introduced, excessive protein consumption is discouraged and well balanced Mediterranean diet is recommended. In advanced stages, additional intake of low-protein products such as bread or pasta is recommended for similar reasons.[10] To minimize interaction with proteins, levodopa should be taken 30 minutes before meals.[10] At the same time, regimens for PD restrict proteins during breakfast and lunch, allowing protein intake in the evening.[10]
Repetitive transcranial magnetic stimulation temporarily improves levodopa-induced dyskinesias.[102] Its usefulness in PD is an open research topic,[103] although recent studies have shown no effect by rTMS.[104] Several nutrients have been proposed as possible treatments; however there is no evidence that vitamins or food additives improve symptoms.[105] There is no evidence to substantiate that acupuncture and practice of Qigong, or T'ai chi, have any effect on the course of the disease or symptoms. Further research on the viability of Tai chi for balance or motor skills are necessary.[106][107][108] Fava beans and velvet beans are natural sources of levodopa and are eaten by many people with PD. While they have shown some effectiveness in clinical trials,[109] their intake is not free of risks. Life-threatening adverse reactions have been described, such as the neuroleptic malignant syndrome.[110][111]
Prognosis

|
no data
< 5
5–12.5
12.5–20
20–27.5
27.5–35
35–42.5
|
42.5–50
50–57.5
57.5–65
65–72.5
72.5–80
> 80
|
PD invariably progresses with time. A severity rating method known as the Unified Parkinson's disease rating scale (UPDRS) is the most commonly used metric for clinical study. A modified version known as the MDS-UPDRS is also sometimes used. An older scaling method known as the Hoehn and Yahr scale (originally published in 1967), and a similar scale known as the Modified Hoehn and Yahr scale, have also been commonly used. The Hoehn and Yahr scale defines five basic stages of progression.
Motor symptoms, if not treated, advance aggressively in the early stages of the disease and more slowly later. Untreated, individuals are expected to lose independent ambulation after an average of eight years and be bedridden after ten years.[112] However, it is uncommon to find untreated people nowadays. Medication has improved the prognosis of motor symptoms, while at the same time it is a new source of disability, because of the undesired effects of levodopa after years of use.[112] In people taking levodopa, the progression time of symptoms to a stage of high dependency from caregivers may be over 15 years.[112] However, it is hard to predict what course the disease will take for a given individual.[112] Age is the best predictor of disease progression.[55] The rate of motor decline is greater in those with less impairment at the time of diagnosis, while cognitive impairment is more frequent in those who are over 70 years of age at symptom onset.[55]
Since current therapies improve motor symptoms, disability at present is mainly related to non-motor features of the disease.[55] Nevertheless, the relationship between disease progression and disability is not linear. Disability is initially related to motor symptoms.[112] As the disease advances, disability is more related to motor symptoms that do not respond adequately to medication, such as swallowing/speech difficulties, and gait/balance problems; and also to levodopa-induced complications, which appear in up to 50% of individuals after 5 years of levodopa usage.[112] Finally, after ten years most people with the disease have autonomic disturbances, sleep problems, mood alterations and cognitive decline.[112] All of these symptoms, especially cognitive decline, greatly increase disability.[55][112]
The life expectancy of people with PD is reduced.[112] Mortality ratios are around twice those of unaffected people.[112] Cognitive decline and dementia, old age at onset, a more advanced disease state and presence of swallowing problems are all mortality risk factors. On the other hand, a disease pattern mainly characterized by tremor as opposed to rigidity predicts an improved survival.[112] Death from aspiration pneumonia is twice as common in individuals with PD as in the healthy population.[112]
In 2013 PD resulted in about 103,000 deaths globally, up from 44,000 deaths in 1990.[113] The death rate increased from an average of 1.5 to 1.8 per 100,000 during that time.[113]
Epidemiology

PD is the second most common neurodegenerative disorder after Alzheimer's disease and affects approximately seven million people globally and one million people in the United States.[36][69] The proportion in a population at a given time is about 0.3% in industrialized countries. PD is more common in the elderly and rates rises from 1% in those over 60 years of age to 4% of the population over 80.[69] The mean age of onset is around 60 years, although 5–10% of cases, classified as young onset PD, begin between the ages of 20 and 50.[22] PD may be less prevalent in those of African and Asian ancestry, although this finding is disputed.[69] Some studies have proposed that it is more common in men than women, but others failed to detect any differences between the two sexes.[69] The number of new cases per year of PD is between 8 and 18 per 100,000 person–years.[69]
Many risk factors and protective factors have been proposed, sometimes in relation to theories concerning possible mechanisms of the disease, however, none have been conclusively related to PD by empirical evidence. When epidemiological studies have been carried out in order to test the relationship between a given factor and PD, they have often been flawed and their results have in some cases been contradictory.[69] The most frequently replicated relationships are an increased risk of PD in those exposed to pesticides, and a reduced risk in smokers.[69]
History

Several early sources, including an Egyptian papyrus, an Ayurvedic medical treatise, the Bible, and Galen's writings, describe symptoms resembling those of PD.[114] After Galen there are no references unambiguously related to PD until the 17th century.[114] In the 17th and 18th centuries, several authors wrote about elements of the disease, including Sylvius, Gaubius, Hunter and Chomel.[114][115][116]
In 1817 an English doctor, James Parkinson, published his essay reporting six cases of paralysis agitans.[15] An Essay on the Shaking Palsy described the characteristic resting tremor, abnormal posture and gait, paralysis and diminished muscle strength, and the way that the disease progresses over time.[13][117] Early neurologists who made further additions to the knowledge of the disease include Trousseau, Gowers, Kinnier Wilson and Erb, and most notably Jean-Martin Charcot, whose studies between 1868 and 1881 were a landmark in the understanding of the disease.[15] Among other advances, he made the distinction between rigidity, weakness and bradykinesia.[15] He also championed the renaming of the disease in honor of James Parkinson.[15]
In 1912 Frederic Lewy described microscopic particles in affected brains, later named "Lewy bodies".[15] In 1919 Konstantin Tretiakoff reported that the substantia nigra was the main cerebral structure affected, but this finding was not widely accepted until it was confirmed by further studies published by Rolf Hassler in 1938.[15] The underlying biochemical changes in the brain were identified in the 1950s, due largely to the work of Arvid Carlsson on the neurotransmitter dopamine and Oleh Hornykiewicz on its role on PD.[118] In 1997, alpha-synuclein was found to be the main component of Lewy bodies by Spillantini, Trojanowski, Goedert and others.[56]
Anticholinergics and surgery (lesioning of the corticospinal pathway or some of the basal ganglia structures) were the only treatments until the arrival of levodopa, which reduced their use dramatically.[115][119] Levodopa was first synthesized in 1911 by Casimir Funk, but it received little attention until the mid 20th century.[118] It entered clinical practice in 1967 and brought about a revolution in the management of PD.[118][120] By the late 1980s deep brain stimulation introduced by Alim Louis Benabid and colleagues at Grenoble, France, emerged as a possible treatment.[121]
Society and culture
Cost

The costs of PD to society are high, but precise calculations are difficult due to methodological issues in research and differences between countries.[122] The annual cost in the UK is estimated to be between 449 million and 3.3 billion pounds, while the cost per patient per year in the U.S. is probably around $10,000 and the total burden around 23 billion dollars.[122] The largest share of direct cost comes from inpatient care and nursing homes, while the share coming from medication is substantially lower.[122] Indirect costs are high, due to reduced productivity and the burden on caregivers.[122] In addition to economic costs, PD reduces quality of life of those with the disease and their caregivers.[122]
Advocacy
11 April, the birthday of James Parkinson, has been designated as World Parkinson's Day.[15] A red tulip was chosen by international organizations as the symbol of the disease in 2005: it represents the James Parkinson Tulip cultivar, registered in 1981 by a Dutch horticulturalist.[123] Advocacy organizations include the National Parkinson Foundation, which has provided more than $180 million in care, research and support services since 1982,[124] Parkinson's Disease Foundation, which has distributed more than $115 million for research and nearly $50 million for education and advocacy programs since its founding in 1957 by William Black;[125][126] the American Parkinson Disease Association, founded in 1961;[127] and the European Parkinson's Disease Association, founded in 1992.[128]
Notable cases

Actor Michael J. Fox has PD and has greatly increased the public awareness of the disease.[16] After diagnosis, Fox embraced his Parkinson's in television roles, sometimes acting without medication, in order to further illustrate the effects of the condition. He has written two autobiographies in which his fight against the disease plays a major role,[129] and appeared before the United States Congress without medication to illustrate the effects of the disease.[129] The Michael J. Fox Foundation aims to develop a cure for Parkinson's disease.[129] Fox received an honorary doctorate in medicine from Karolinska Institutet for his contributions to research in Parkinson's disease.[130]
Professional cyclist and Olympic medalist Davis Phinney, who was diagnosed with young onset Parkinson's at age 40, started the Davis Phinney Foundation in 2004 to support Parkinson's research, focusing on quality of life for people with the disease.[17][131][132]
Boxer Muhammad Ali showed signs of Parkinson's when he was 38, but was not diagnosed until he was 42, and has been called the "world's most famous Parkinson's patient".[18] Whether he had PD or parkinsonism related to boxing is unresolved.[133][134]
Research
There is little prospect of significant new PD treatments in the near future.[135] Currently active research directions include the search for new animal models of the disease and studies of the potential usefulness of gene therapy, stem cell transplants and neuroprotective agents.[55]
Animal models
PD is not known to occur naturally in any species other than humans, although animal models which show some features of the disease are used in research. The appearance of parkinsonism in a group of drug addicts in the early 1980s who consumed a contaminated batch of the synthetic opiate MPPP led to the discovery of the chemical MPTP as an agent that causes parkinsonism in non-human primates as well as in humans.[136] Other predominant toxin-based models employ the insecticide rotenone, the herbicide paraquat and the fungicide maneb.[137] Models based on toxins are most commonly used in primates. Transgenic rodent models that replicate various aspects of PD have been developed.[138] Using the neurotoxin 6-hydroxydopamine, also known as 6-OHDA, it creates a model of Parkinson's disease in rats by targeting and destroying dopaminergic neurons in the nigrostriatal pathway when injected into the substantia nigra.[139]
Gene therapy
Gene therapy typically involves the use of a non-infectious virus, i.e., a viral vector such as the adeno-associated virus, to shuttle genetic material into a part of the brain. The gene used leads to the production of an enzyme that helps to manage PD symptoms or protects the brain from further damage.[55][140] In 2010 there were four clinical trials using gene therapy in PD.[55] There have not been important adverse effects in these trials although the clinical usefulness of gene therapy is still unknown.[55] One of these reported positive results in 2011,[141] but the company filed for bankruptcy in March 2012.[142]
Neuroprotective treatments

Investigations on neuroprotection are at the forefront of PD research. Several molecules have been proposed as potential treatments.[55] However, none of them have been conclusively demonstrated to reduce degeneration.[55] Agents currently under investigation include anti-apoptotics (omigapil, CEP-1347), antiglutamatergics, monoamine oxidase inhibitors (selegiline, rasagiline), promitochondrials (coenzyme Q10, creatine), calcium channel blockers (isradipine) and growth factors (GDNF).[55] Preclinical research also targets alpha-synuclein.[135] A vaccine that primes the human immune system to destroy alpha-synuclein, PD01A (developed by Austrian company, Affiris), has entered clinical trials in humans.[143]
Neural transplantation
Since early in the 1980s, fetal, porcine, carotid or retinal tissues have been used in cell transplants, in which dissociated cells are injected into the substantia nigra in the hope that they will incorporate themselves into the brain in a way that replaces the dopamine-producing cells that have been lost.[55] Although there was initial evidence of mesencephalic dopamine-producing cell transplants being beneficial, double-blind trials to date indicate that cell transplants produce no long-term benefit.[55] An additional significant problem was the excess release of dopamine by the transplanted tissue, leading to dystonias.[144] Stem cell transplants are a recent research target, because stem cells are easy to manipulate and stem cells transplanted into the brains of rodents and monkeys have been found to survive and reduce behavioral abnormalities.[55][145] Nevertheless, use of fetal stem cells is controversial.[55] It has been proposed that effective treatments may be developed in a less controversial way by use of induced pluripotent stem cells taken from adults.[55]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "Parkinson's Disease Information Page". NINDS. June 30, 2016. Retrieved 18 July 2016.
- 1 2 3 4 5 6 7 8 9 10 Sveinbjornsdottir, S (11 July 2016). "The clinical symptoms of Parkinson's disease.". Journal of Neurochemistry. 139: 318–324. PMID 27401947. doi:10.1111/jnc.13691.
- 1 2 Carroll, William M. (2016). International Neurology. John Wiley & Sons. p. 188. ISBN 9781118777367.
- 1 2 3 4 5 6 7 8 9 10 11 Kalia, LV; Lang, AE (29 August 2015). "Parkinson's disease.". Lancet. 386 (9996): 896–912. PMID 25904081. doi:10.1016/s0140-6736(14)61393-3.
- ↑ Ferri, Fred F. (2010). Ferri's differential diagnosis : a practical guide to the differential diagnosis of symptoms, signs, and clinical disorders (2nd ed. ed.). Philadelphia, PA: Elsevier/Mosby. p. Chapter P. ISBN 0323076998.
- 1 2 GBD 2015 Disease and Injury Incidence and Prevalence, Collaborators. (8 October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015.". Lancet. 388 (10053): 1545–1602. PMC 5055577
 . PMID 27733282. doi:10.1016/S0140-6736(16)31678-6.
. PMID 27733282. doi:10.1016/S0140-6736(16)31678-6. - 1 2 GBD 2015 Mortality and Causes of Death, Collaborators. (8 October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015.". Lancet. 388 (10053): 1459–1544. PMC 5388903
 . PMID 27733281. doi:10.1016/s0140-6736(16)31012-1.
. PMID 27733281. doi:10.1016/s0140-6736(16)31012-1. - ↑ Jones, H. Royden (2013). The Netter collection of medical illustrations. a compilation of paintings (2nd ed.). Philadelphia, PA: Saunders Elsevier. p. 161. ISBN 9781455733873.
- ↑ Barranco Quintana, JL; Allam, MF; Del Castillo, AS; Navajas, RF (February 2009). "Parkinson's disease and tea: a quantitative review.". Journal of the American College of Nutrition. 28 (1): 1–6. PMID 19571153. doi:10.1080/07315724.2009.10719754.
- 1 2 3 4 5 6 7 8 9 10 11 Barichella M, Cereda E, Pezzoli G (October 2009). "Major nutritional issues in the management of Parkinson's disease". Mov. Disord. 24 (13): 1881–92. PMID 19691125. doi:10.1002/mds.22705.
- 1 2 3 Ahlskog, JE (19 July 2011). "Does vigorous exercise have a neuroprotective effect in Parkinson disease?". Neurology. 77 (3): 288–94. PMC 3136051
 . PMID 21768599. doi:10.1212/wnl.0b013e318225ab66.
. PMID 21768599. doi:10.1212/wnl.0b013e318225ab66. - ↑ Mosley, Anthony D. (2010). The encyclopedia of Parkinson's disease (2nd ed.). New York: Facts on File. p. 89. ISBN 9781438127491.
- 1 2 "An Essay on the Shaking Palsy".
- ↑ Shulman JM, De Jager PL, Feany MB (February 2011) [25 October 2010]. "Parkinson's disease: genetics and pathogenesis.". Annual Review of Pathology. 6: 193–222. PMID 21034221. doi:10.1146/annurev-pathol-011110-130242.
- 1 2 3 4 5 6 7 8 Lees AJ (September 2007). "Unresolved issues relating to the shaking palsy on the celebration of James Parkinson's 250th birthday". Mov. Disord. 22 (Suppl 17): S327–34. PMID 18175393. doi:10.1002/mds.21684.
- 1 2 Davis P (3 May 2007). "Michael J. Fox". The TIME 100. Time. Retrieved 2 April 2011.
- 1 2 Macur, Juliet (26 March 2008). "For the Phinney Family, a Dream and a Challenge". The New York Times. Retrieved 25 May 2013.
About 1.5 million Americans have received a diagnosis of Parkinson's disease, but only 5 to 10 percent learn of it before age 40, according to the National Parkinson Foundation. Davis Phinney was among the few.
- 1 2 Brey RL (April 2006). "Muhammad Ali's Message: Keep Moving Forward". Neurology Now. American Academy of Neurology. 2 (2): 8. doi:10.1097/01222928-200602020-00003. Archived from the original on 27 September 2011. Retrieved 2 April 2011.
- ↑ Ling, Helen; Massey, Luke A.; Lees, Andrew J.; Brown, Peter; Day, Brian L. (April 2012). "Hypokinesia without decrement distinguishes progressive supranuclear palsy from Parkinson's disease". Brain: A Journal of Neurology. 135 (Pt 4): 1141–1153. ISSN 1460-2156. PMC 3326257
 . PMID 22396397. doi:10.1093/brain/aws038.
. PMID 22396397. doi:10.1093/brain/aws038. - ↑ "Parkinson’s Disease vs. Parkinsonism" (PDF). National Parkinson Foundation. Retrieved 22 June 2017.
- ↑ "Queen Square Brain Bank diagnostic criteria for Parkinson's disease". Retrieved 22 June 2017.
- 1 2 3 4 5 6 7 8 9 10 Samii A, Nutt JG, Ransom BR (29 May 2004). "Parkinson's disease". Lancet. 363 (9423): 1183–93. PMID 15172778. doi:10.1016/S0140-6736(04)16305-8.
- ↑ Schrag A (2007). "Epidemiology of movement disorders". In Tolosa E, Jankovic JJ. Parkinson's disease and movement disorders. Hagerstown, Maryland: Lippincott Williams & Wilkins. pp. 50–66. ISBN 0-7817-7881-6.
- ↑ Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C (July 2010) [18 May 2010]. "Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update". Human Mutation. 31 (7): 763–80. PMC 3056147
 . PMID 20506312. doi:10.1002/humu.21277.
. PMID 20506312. doi:10.1002/humu.21277. - ↑ Galpern WR, Lang AE (March 2006) [17 February 2006]. "Interface between tauopathies and synucleinopathies: a tale of two proteins". Annals of Neurology. 59 (3): 449–58. PMID 16489609. doi:10.1002/ana.20819.
- 1 2 Aarsland D, Londos E, Ballard C (April 2009) [28 January 2009]. "Parkinson's disease dementia and dementia with Lewy bodies: different aspects of one entity". Int. Psychogeriatr. 21 (2): 216–19. PMID 19173762. doi:10.1017/S1041610208008612.
- ↑ Photo by Arthur Londe from Nouvelle Iconographie de la Salpètrière, vol. 5, p. 226
- ↑ Charcot, Jean-Martin; Sigerson, George (1879). Lectures on the diseases of the nervous system (Second ed.). Philadelphia: Henry C. Lea. p. 113.
The strokes forming the letters are very irregular and sinuous, whilst the irregularities and sinuosities are of a very limited width. (...) the down-strokes are all, with the exception of the first letter, made with comparative firmness and are, in fact, nearly normal – the finer up-strokes, on the contrary, are all tremulous in appearance (...).
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Jankovic J (April 2008). "Parkinson's disease: clinical features and diagnosis". Journal of Neurology, Neurosurgery, and Psychiatry. 79 (4): 368–76. PMID 18344392. doi:10.1136/jnnp.2007.131045.
- 1 2 Cooper G, Eichhorn G, Rodnitzky RL (2008). "Parkinson's disease". In Conn PM. Neuroscience in medicine. Totowa, NJ: Humana Press. pp. 508–12. ISBN 978-1-60327-454-8.
- ↑ Lees, Andrew J.; Hardy, John; Revesz, Tamas (2009-06-13). "Parkinson's disease". Lancet. 373 (9680): 2055–2066. ISSN 1474-547X. PMID 19524782. doi:10.1016/S0140-6736(09)60492-X.
- ↑ Banich MT, Compton RJ (2011). "Motor control". Cognitive neuroscience. Belmont, CA: Wadsworth, Cengage learning. pp. 108–44. ISBN 0-8400-3298-6.
- ↑ Longmore M, Wilkinson IB, Turmezei T, Cheung CK (4 January 2007). Oxford Handbook of Clinical Medicine. Oxford University Press. p. 486. ISBN 978-0-19-856837-7.
- ↑ Fung VS, Thompson PD (2007). "Rigidity and spasticity". In Tolosa E, Jankovic. Parkinson's disease and movement disorders. Hagerstown, MD: Lippincott Williams & Wilkins. pp. 504–13. ISBN 0-7817-7881-6.
- ↑ O'Sullivan SB, Schmitz TJ (2007). "Parkinson's Disease". Physical Rehabilitation (5th ed.). Philadelphia: F.A. Davis. pp. 856–57.
- 1 2 Yao, S.C.; Hart, A.D.; Terzella, M.J. (May 2013). "An evidence-based osteopathic approach to Parkinson disease". Osteopathic Family Physician. 5 (3): 96–101. doi:10.1016/j.osfp.2013.01.003.
- ↑ Mark Hallett; Werner Poewe (13 October 2008). Therapeutics of Parkinson's Disease and Other Movement Disorders. John Wiley & Sons. p. 417. ISBN 978-0-470-71400-3.
- ↑ Hoehn, M. M.; Yahr, M. D. (May 1967). "Parkinsonism: onset, progression and mortality". Neurology. 17 (5): 427–442. ISSN 0028-3878. PMID 6067254.
- ↑ Rajesh Pahwa; Kelly E. Lyons (25 March 2003). Handbook of Parkinson's Disease, Third Edition. CRC Press. p. 76. ISBN 978-0-203-91216-4.
- 1 2 3 4 5 6 7 Caballol N, Martí MJ, Tolosa E (September 2007). "Cognitive dysfunction and dementia in Parkinson disease". Movement Disorders. 22 (Suppl 17): S358–66. PMID 18175397. doi:10.1002/mds.21677.
- 1 2 3 Parker KL, Lamichhane D, Caetano MS, Narayanan NS (October 2013). "Executive dysfunction in Parkinson's disease and timing deficits". Front. Integr. Neurosci. 7: 75. PMC 3813949
 . PMID 24198770. doi:10.3389/fnint.2013.00075.
. PMID 24198770. doi:10.3389/fnint.2013.00075. - ↑ Garcia-Ptacek, Sara; Kramberger, Milica G. (September 2016). "Parkinson Disease and Dementia". Journal of Geriatric Psychiatry and Neurology. 29 (5): 261–270. ISSN 0891-9887. PMID 27502301. doi:10.1177/0891988716654985.
- ↑ Shergill SS, Walker Z, Le Katona C (October 1998). "A preliminary investigation of laterality in Parkinson's disease and susceptibility to psychosis". J. Neurol. Neurosurg. Psychiatr. 65 (4): 610–11. PMC 2170290
 . PMID 9771806. doi:10.1136/jnnp.65.4.610.
. PMID 9771806. doi:10.1136/jnnp.65.4.610. - ↑ Friedman JH (June 2010). "Parkinson's disease psychosis 2010: A review article". Parkinsonism Relat. Disord. 16 (9): 553–60. PMID 20538500. doi:10.1016/j.parkreldis.2010.05.004.
- ↑ Eun, Kim, Young; S., Jeon, Beom (2014-01-01). "Clinical Implication of REM Sleep Behavior Disorder in Parkinson's Disease". Journal of Parkinson's Disease. 4 (2). ISSN 1877-7171. doi:10.3233/jpd-130293.
- ↑ Noyce, Alastair J.; Bestwick, Jonathan P.; Silveira-Moriyama, Laura; Hawkes, Christopher H.; Giovannoni, Gavin; Lees, Andrew J.; Schrag, Anette (December 2012). "Meta-analysis of early nonmotor features and risk factors for Parkinson disease". Annals of Neurology. 72 (6): 893–901. ISSN 1531-8249. PMC 3556649
 . PMID 23071076. doi:10.1002/ana.23687.
. PMID 23071076. doi:10.1002/ana.23687. - ↑ Chahine, Lama M.; Stern, Matthew B.; Chen-Plotkin, Alice (January 2014). "Blood-based biomarkers for Parkinson's disease". Parkinsonism & Related Disorders. 20 Suppl 1: S99–103. ISSN 1873-5126. PMC 4070332
 . PMID 24262199. doi:10.1016/S1353-8020(13)70025-7.
. PMID 24262199. doi:10.1016/S1353-8020(13)70025-7. - 1 2 3 4 5 Lesage S, Brice A (April 2009). "Parkinson's disease: from monogenic forms to genetic susceptibility factors". Hum. Mol. Genet. 18 (R1): R48–59. PMID 19297401. doi:10.1093/hmg/ddp012.
- 1 2 Kalia, Lorraine V.; Lang, Anthony E. (2015-08-29). "Parkinson's disease". Lancet. 386 (9996): 896–912. ISSN 1474-547X. PMID 25904081. doi:10.1016/S0140-6736(14)61393-3.
- 1 2 3 4 Davie CA (2008). "A review of Parkinson's disease". Br. Med. Bull. 86 (1): 109–27. PMID 18398010. doi:10.1093/bmb/ldn013.
- ↑ Gan-Or, Ziv; Dion, Patrick A.; Rouleau, Guy A. (2 September 2015). "Genetic perspective on the role of the autophagy-lysosome pathway in Parkinson disease". Autophagy. 11 (9): 1443–57. PMC 4590678
 . PMID 26207393. doi:10.1080/15548627.2015.1067364.
. PMID 26207393. doi:10.1080/15548627.2015.1067364. - 1 2 Dickson DV (2007). "Neuropathology of movement disorders". In Tolosa E, Jankovic JJ. Parkinson's disease and movement disorders. Hagerstown, MD: Lippincott Williams & Wilkins. pp. 271–83. ISBN 0-7817-7881-6.
- ↑ Jubault T, Brambati SM, Degroot C (2009). Gendelman, Howard E., ed. "Regional brain stem atrophy in idiopathic Parkinson's disease detected by anatomical MRI". PLoS ONE. 4 (12): e8247. Bibcode:2009PLoSO...4.8247J. PMC 2784293
 . PMID 20011063. doi:10.1371/journal.pone.0008247.
. PMID 20011063. doi:10.1371/journal.pone.0008247. - 1 2 3 4 5 6 Obeso JA, Rodríguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M (2008). "Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease". Mov. Disord. 23 (Suppl 3): S548–59. PMID 18781672. doi:10.1002/mds.22062.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, Hirsch EC, Farrer M, Schapira AH, Halliday G (May 2010). "Missing pieces in the Parkinson's disease puzzle". Nat. Med. 16 (6): 653–61. PMID 20495568. doi:10.1038/nm.2165.
- 1 2 3 Schulz-Schaeffer WJ (August 2010). "The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia". Acta Neuropathol. 120 (2): 131–43. PMC 2892607
 . PMID 20563819. doi:10.1007/s00401-010-0711-0.
. PMID 20563819. doi:10.1007/s00401-010-0711-0. - ↑ Hirsch EC (December 2009). "Iron transport in Parkinson's disease". Parkinsonism Relat. Disord. 15 (Suppl 3): S209–11. PMID 20082992. doi:10.1016/S1353-8020(09)70816-8.
- 1 2 The National Collaborating Centre for Chronic Conditions, ed. (2006). "Diagnosing Parkinson's Disease". Parkinson's Disease. London: Royal College of Physicians. pp. 29–47. ISBN 1-86016-283-5.
- ↑ Poewe W, Wenning G (November 2002). "The differential diagnosis of Parkinson's disease". Eur. J. Neurol. 9 (Suppl 3): 23–30. PMID 12464118. doi:10.1046/j.1468-1331.9.s3.3.x.
- ↑ Gibb, W R; Lees, A J (June 1988). "The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease.". Journal of Neurology, Neurosurgery, and Psychiatry. 51 (6): 745–752. ISSN 0022-3050. PMC 1033142
 . PMID 2841426.
. PMID 2841426. - ↑ Rizzo, Giovanni; Copetti, Massimiliano; Arcuti, Simona; Martino, Davide; Fontana, Andrea; Logroscino, Giancarlo (2016-02-09). "Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis". Neurology. 86 (6): 566–576. ISSN 1526-632X. PMID 26764028. doi:10.1212/WNL.0000000000002350.
- ↑ Postuma, Ronald B.; Berg, Daniela; Stern, Matthew; Poewe, Werner; Olanow, C. Warren; Oertel, Wolfgang; Obeso, José; Marek, Kenneth; Litvan, Irene (October 2015). "MDS clinical diagnostic criteria for Parkinson's disease". Movement Disorders: Official Journal of the Movement Disorder Society. 30 (12): 1591–1601. ISSN 1531-8257. PMID 26474316. doi:10.1002/mds.26424.
- ↑ Berg, Daniela; Postuma, Ronald B.; Adler, Charles H.; Bloem, Bastiaan R.; Chan, Piu; Dubois, Bruno; Gasser, Thomas; Goetz, Christopher G.; Halliday, Glenda (October 2015). "MDS research criteria for prodromal Parkinson's disease". Movement Disorders: Official Journal of the Movement Disorder Society. 30 (12): 1600–1611. ISSN 1531-8257. PMID 26474317. doi:10.1002/mds.26431.
- 1 2 3 4 Brooks DJ (April 2010). "Imaging approaches to Parkinson disease". J. Nucl. Med. 51 (4): 596–609. PMID 20351351. doi:10.2967/jnumed.108.059998.
- ↑ Schwarz, ST; Afzal, M; Morgan, PS; Bajaj, N; Gowland, PA; Auer, DP (2014). "The 'swallow tail' appearance of the healthy nigrosome - a new accurate test of Parkinson's disease: a case-control and retrospective cross-sectional MRI study at 3T.". PLOS ONE. 9 (4): e93814. PMC 3977922
 . PMID 24710392. doi:10.1371/journal.pone.0093814.
. PMID 24710392. doi:10.1371/journal.pone.0093814. - ↑ Mahlknecht, Philipp; Krismer, Florian; Poewe, Werner; Seppi, Klaus (2017-02-02). "Meta-analysis of dorsolateral nigral hyperintensity on magnetic resonance imaging as a marker for Parkinson's disease". Movement Disorders: Official Journal of the Movement Disorder Society. PMID 28151553. doi:10.1002/mds.26932.
- ↑ Costa J, Lunet N, Santos C, Santos J, Vaz-Carneiro A (2010). "Caffeine exposure and the risk of Parkinson's disease: a systematic review and meta-analysis of observational studies". J. Alzheimers Dis. 20 (Suppl 1): S221–38. PMID 20182023. doi:10.3233/JAD-2010-091525.
- ↑ Ma, Chaoran; Liu, Yesong; Neumann, Samantha; Gao, Xiang (2017). "Nicotine from cigarette smoking and diet and Parkinson disease: a review". Translational Neurodegeneration. 6: 18. ISSN 2047-9158. PMC 5494127
 . PMID 28680589. doi:10.1186/s40035-017-0090-8.
. PMID 28680589. doi:10.1186/s40035-017-0090-8. - 1 2 3 4 5 6 7 8 9 de Lau LM, Breteler MM (June 2006). "Epidemiology of Parkinson's disease". Lancet Neurol. 5 (6): 525–35. PMID 16713924. doi:10.1016/S1474-4422(06)70471-9.
- ↑ Gagne JJ, Power MC (March 2010). "Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis". Neurology. 74 (12): 995–1002. PMC 2848103
 . PMID 20308684. doi:10.1212/WNL.0b013e3181d5a4a3.
. PMID 20308684. doi:10.1212/WNL.0b013e3181d5a4a3. - ↑ Connolly, Barbara S.; Lang, Anthony E. (23–30 April 2014). "Pharmacological treatment of Parkinson disease: a review". JAMA. 311 (16): 1670–1683. ISSN 1538-3598. PMID 24756517. doi:10.1001/jama.2014.3654.
- ↑ "The non-motor and non-dopaminergic fratures of PD". Parkinson's Disease : Non-Motor and Non-Dopaminergic Features. Olanow, C. Warren., Stocchi, Fabrizio., Lang, Anthony E. Wiley-Blackwell. 2011. ISBN 1405191856. OCLC 743205140.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 The National Collaborating Centre for Chronic Conditions, ed. (2006). "Symptomatic pharmacological therapy in Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 59–100. ISBN 1-86016-283-5.
- ↑ Zhang, Jinglin; Tan, Louis Chew-Seng (2016). "Revisiting the Medical Management of Parkinson's Disease: Levodopa versus Dopamine Agonist". Current Neuropharmacology. 14 (4): 356–363. ISSN 1875-6190. PMC 4876591
 . PMID 26644151. doi:10.2174/1570159X14666151208114634.
. PMID 26644151. doi:10.2174/1570159X14666151208114634. - 1 2 Pedrosa, David J.; Timmermann, Lars (2013). "Review: management of Parkinson's disease". Neuropsychiatric Disease and Treatment. 9: 321–340. ISSN 1176-6328. PMC 3592512
 . PMID 23487540. doi:10.2147/NDT.S32302.
. PMID 23487540. doi:10.2147/NDT.S32302. - ↑ The National Collaborating Centre for Chronic Conditions, ed. (2006). "Palliative care in Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 147–51. ISBN 1-86016-283-5.
- ↑ Maria., Nord, (2017). Levodopa pharmacokinetics -from stomach to brain A study on patients with Parkinson's disease. Linköping: Linköping University Electronic Press. p. 10. ISBN 9789176855577. OCLC 993068595.
- 1 2 Oertel, Wolfgang H. (2017-03-13). "Recent advances in treating Parkinson’s disease". F1000Research. 6. ISSN 2046-1402. PMC 5357034
 . PMID 28357055. doi:10.12688/f1000research.10100.1.
. PMID 28357055. doi:10.12688/f1000research.10100.1. - ↑ Aquino, Camila Catherine; Fox, Susan H. (January 2015). "Clinical spectrum of levodopa-induced complications". Movement Disorders: Official Journal of the Movement Disorder Society. 30 (1): 80–89. ISSN 1531-8257. PMID 25488260. doi:10.1002/mds.26125.
- ↑ Goldenberg MM (October 2008). "Medical management of Parkinson's disease". P & T. 33 (10): 590–606. PMC 2730785
 . PMID 19750042.
. PMID 19750042. - ↑ Ceravolo R, Frosini D, Rossi C, Bonuccelli U (December 2009). "Impulse control disorders in Parkinson's disease: definition, epidemiology, risk factors, neurobiology and management". Parkinsonism Relat. Disord. 15 (Suppl 4): S111–15. PMID 20123548. doi:10.1016/S1353-8020(09)70847-8.
- ↑ Tolosa E, Katzenschlager R (2007). "Pharmacological management of Parkinson's disease". In Tolosa E, Jankovic JJ. Parkinson's disease and movement disorders. Hagerstwon, MD: Lippincott Williams & Wilkins. pp. 110–45. ISBN 0-7817-7881-6.
- 1 2 The National Collaborating Centre for Chronic Conditions, ed. (2006). "Non-motor features of Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 113–33. ISBN 1-86016-283-5.
- ↑ Hasnain M, Vieweg WV, Baron MS, Beatty-Brooks M, Fernandez A, Pandurangi AK (July 2009). "Pharmacological management of psychosis in elderly patients with parkinsonism". Am. J. Med. 122 (7): 614–22. PMID 19559160. doi:10.1016/j.amjmed.2009.01.025.
- 1 2 3 4 The National Collaborating Centre for Chronic Conditions, ed. (2006). "Surgery for Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 101–11. ISBN 1-86016-283-5.
- ↑ Bronstein, M.; et al. (February 2011). "Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues". Arch. Neurol. 68 (2): 165. PMID 20937936. doi:10.1001/archneurol.2010.260.
- 1 2 3 4 5 The National Collaborating Centre for Chronic Conditions, ed. (2006). "Other key interventions". Parkinson's Disease. London: Royal College of Physicians. pp. 135–46. ISBN 1-86016-283-5.
- 1 2 Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL (April 2008). "The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis". Mov. Disord. 23 (5): 631–40. PMID 18181210. doi:10.1002/mds.21922.
- ↑ Dereli EE, Yaliman A (April 2010). "Comparison of the effects of a physiotherapist-supervised exercise programme and a self-supervised exercise programme on quality of life in patients with Parkinson's disease". Clin Rehabil. 24 (4): 352–62. PMID 20360152. doi:10.1177/0269215509358933.
- ↑ O'Sullivan & Schmitz 2007, pp. 873, 876
- ↑ O'Sullivan & Schmitz 2007, p. 879
- ↑ O'Sullivan & Schmitz 2007, p. 877
- ↑ O'Sullivan & Schmitz 2007, p. 880
- ↑ Fox CM, Ramig LO, Ciucci MR, Sapir S, McFarland DH, Farley BG (November 2006). "The science and practice of LSVT/LOUD: neural plasticity-principled approach to treating individuals with Parkinson disease and other neurological disorders". Semin. Speech. Lang. 27 (4): 283–99. PMID 17117354. doi:10.1055/s-2006-955118.
- ↑ Dixon L, Duncan D, Johnson P, Kirkby L, O'Connell H, Taylor H, Deane KH (2007). Deane, Katherine, ed. "Occupational therapy for patients with Parkinson's disease". Cochrane Database of Systematic Reviews (3): CD002813. PMID 17636709. doi:10.1002/14651858.CD002813.pub2.
- ↑ Ferrell B, Connor SR, Cordes A, Dahlin CM, Fine PG, Hutton N, Leenay M, Lentz J, Person JL, Meier DE, Zuroski K; National Consensus Project for Quality Palliative Care Task Force Members (2007). "The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum". J Pain Symptom Manage. 33 (6): 737–44. PMID 17531914. doi:10.1016/j.jpainsymman.2007.02.024.
- 1 2 Lorenzl S, Nübling G, Perrar KM, Voltz R (2013). "Palliative treatment of chronic neurologic disorders". Handb Clin Neurol. Handbook of Clinical Neurology. 118: 133–39. ISBN 9780444535016. PMID 24182372. doi:10.1016/B978-0-444-53501-6.00010-X.
- 1 2 Ghoche R (2012). "The conceptual framework of palliative care applied to advanced Parkinson's disease.". Parkinsonism Relat Disord. 18 (Suppl 3): S2–5. PMID 22771241. doi:10.1016/j.parkreldis.2012.06.012.
- 1 2 3 Wilcox SK (2010). "Extending palliative care to patients with Parkinson's disease". Br J Hosp Med (Lond). 71 (1): 26–30. PMID 20081638. doi:10.12968/hmed.2010.71.1.45969.
- ↑ Moens K, Higginson IJ, Harding R, EURO IMPACT (2014). "Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review". J Pain Symptom Manage. 48 (4): 660–77. PMID 24801658. doi:10.1016/j.jpainsymman.2013.11.009.
- ↑ Casey G (2013). "Parkinson's disease: a long and difficult journey.". Nurs N Z. 19 (7): 20–24. PMID 24195263.
- ↑ Koch G (2010). "rTMS effects on levodopa induced dyskinesias in Parkinson's disease patients: searching for effective cortical targets". Restor. Neurol. Neurosci. 28 (4): 561–68. PMID 20714078. doi:10.3233/RNN-2010-0556.
- ↑ Platz T, Rothwell JC (2010). "Brain stimulation and brain repair – rTMS: from animal experiment to clinical trials – what do we know?". Restor. Neurol. Neurosci. 28 (4): 387–98. PMID 20714064. doi:10.3233/RNN-2010-0570.
- ↑ Arias P, Vivas J, Grieve KL, Cudeiro J (September 2010). "Controlled trial on the effect of 10 days low-frequency repetitive transcranial magnetic stimulation (rTMS) on motor signs in Parkinson's disease". Mov. Disord. 25 (12): 1830–38. PMID 20669300. doi:10.1002/mds.23055.
- ↑ Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz T, Weiner WJ (April 2006). "Practice Parameter: neuroprotective strategies and alternative therapies for Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology". Neurology. 66 (7): 976–82. PMID 16606908. doi:10.1212/01.wnl.0000206363.57955.1b.
- ↑ Lee MS, Lam P, Ernst E (December 2008). "Effectiveness of tai chi for Parkinson's disease: a critical review". Parkinsonism Relat. Disord. 14 (8): 589–94. PMID 18374620. doi:10.1016/j.parkreldis.2008.02.003.
- ↑ Lee MS, Ernst E (January 2009). "Qigong for movement disorders: A systematic review". Mov. Disord. 24 (2): 301–03. PMID 18973253. doi:10.1002/mds.22275.
- ↑ Lee MS, Shin BC, Kong JC, Ernst E (August 2008). "Effectiveness of acupuncture for Parkinson's disease: a systematic review". Mov. Disord. 23 (11): 1505–15. PMID 18618661. doi:10.1002/mds.21993.
- ↑ Katzenschlager R, Evans A, Manson A, Patsalos PN, Ratnaraj N, Watt H, Timmermann L, Van der Giessen R, Lees AJ (2004). "Mucuna pruriens in Parkinson's disease: a double blind clinical and pharmacological study". J. Neurol. Neurosurg. Psychiatr. 75 (12): 1672–77. PMC 1738871
 . PMID 15548480. doi:10.1136/jnnp.2003.028761.
. PMID 15548480. doi:10.1136/jnnp.2003.028761. - ↑ Ladha SS, Walker R, Shill HA (May 2005). "Case of neuroleptic malignant-like syndrome precipitated by abrupt fava bean discontinuance". Mov. Disord. 20 (5): 630–31. PMID 15719433. doi:10.1002/mds.20380.
- ↑ Raguthu L, Varanese S, Flancbaum L, Tayler E, Di Rocco A (October 2009). "Fava beans and Parkinson's disease: useful 'natural supplement' or useless risk?". Eur. J. Neurol. 16 (10): e171. PMID 19678834. doi:10.1111/j.1468-1331.2009.02766.x.
- 1 2 3 4 5 6 7 8 9 10 11 12 Poewe W (December 2006). "The natural history of Parkinson's disease". J. Neurol. 253 (Suppl 7): VII2–6. PMID 17131223. doi:10.1007/s00415-006-7002-7.
- 1 2 GBD 2013 Mortality and Causes of Death, Collaborators (17 December 2014). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013.". Lancet. 385: 117–71. PMC 4340604
 . PMID 25530442. doi:10.1016/S0140-6736(14)61682-2.
. PMID 25530442. doi:10.1016/S0140-6736(14)61682-2. - 1 2 3 García Ruiz PJ (December 2004). "Prehistoria de la enfermedad de Parkinson" [[Prehistory of Parkinson's disease]]. Neurologia (in Spanish). 19 (10): 735–37. PMID 15568171.
- 1 2 Lanska DJ (2010). "Chapter 33: the history of movement disorders". Handb. Clin. Neurol. Handbook of Clinical Neurology. 95: 501–46. ISBN 9780444520098. PMID 19892136. doi:10.1016/S0072-9752(08)02133-7.
- ↑ Koehler PJ, Keyser A (September 1997). "Tremor in Latin texts of Dutch physicians: 16th–18th centuries". Mov. Disord. 12 (5): 798–806. PMID 9380070. doi:10.1002/mds.870120531.
- ↑ Louis ED (November 1997). "The shaking palsy, the first forty-five years: a journey through the British literature". Mov. Disord. 12 (6): 1068–72. PMID 9399240. doi:10.1002/mds.870120638.
- 1 2 3 Fahn S (2008). "The history of dopamine and levodopa in the treatment of Parkinson's disease". Mov. Disord. 23 (Suppl 3): S497–508. PMID 18781671. doi:10.1002/mds.22028.
- ↑ Guridi J, Lozano AM (November 1997). "A brief history of pallidotomy". Neurosurgery. 41 (5): 1169–80; discussion 1180–3. PMID 9361073. doi:10.1097/00006123-199711000-00029.
- ↑ Hornykiewicz O (2002). "L-DOPA: from a biologically inactive amino acid to a successful therapeutic agent". Amino Acids. 23 (1–3): 65–70. PMID 12373520. doi:10.1007/s00726-001-0111-9.
- ↑ Coffey RJ (March 2009). "Deep brain stimulation devices: a brief technical history and review". Artif. Organs. 33 (3): 208–20. PMID 18684199. doi:10.1111/j.1525-1594.2008.00620.x.
- 1 2 3 4 5 Findley LJ (September 2007). "The economic impact of Parkinson's disease". Parkinsonism Relat. Disord. 13 (Suppl): S8–S12. PMID 17702630. doi:10.1016/j.parkreldis.2007.06.003.
- ↑ "Parkinson's – 'the shaking palsy'". GlaxoSmithKline. 1 April 2009. Archived from the original on 14 May 2011.
- ↑ "National Parkinson Foundation – Mission". Archived from the original on 21 December 2010. Retrieved 28 March 2011.
- ↑ "Education: Joy in Giving". Time. 18 January 1960. Retrieved 2 April 2011.
- ↑ "About PDF" (PDF). Parkinson's Disease Foundation. Retrieved 24 July 2016.
- ↑ "American Parkinson Disease Association: Home". American Parkinson Disease Association. Retrieved 9 August 2010.
- ↑ "About EPDA". European Parkinson's Disease Association. 2010. Retrieved 9 August 2010.
- 1 2 3 Brockes E (11 April 2009). "'It's the gift that keeps on taking'". The Guardian. Retrieved 25 October 2010.
- ↑ "Michael J. Fox to be made honorary doctor at Karolinska Institutet". Karolinska Institutet. 5 March 2010. Archived from the original on 30 September 2011. Retrieved 2 April 2011.
- ↑ "Who We Are". Davis Phinney Foundation. Retrieved 18 January 2012.
- ↑ Emanuel, W (19 October 2010). "Davis Phinney foundation launches exercise-focused tools to help people live well with Parkinson's" (PDF) (Press release). Davis Phinney foundation. Retrieved 2 April 2011.
- ↑ Matthews W (April 2006). "Ali's Fighting Spirit". Neurology Now. American Academy of Neurology. 2 (2): 10–23. doi:10.1097/01222928-200602020-00004. Retrieved 2 April 2011.
- ↑ Tauber P (17 July 1988). "Ali: Still Magic". New York Times. Retrieved 2 April 2011.
- 1 2 Dimond PF (16 August 2010). "No New Parkinson Disease Drug Expected Anytime Soon". GEN news highlights. GEN-Genetic Engineering & Biotechnology News.
- ↑ Langston JW, Ballard P, Tetrud JW, Irwin I (February 1983). "Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis". Science. 219 (4587): 979–80. Bibcode:1983Sci...219..979L. PMID 6823561. doi:10.1126/science.6823561.
- ↑ Cicchetti F, Drouin-Ouellet J, Gross RE (September 2009). "Environmental toxins and Parkinson's disease: what have we learned from pesticide-induced animal models?". Trends Pharmacol. Sci. 30 (9): 475–83. PMID 19729209. doi:10.1016/j.tips.2009.06.005.
- ↑ Harvey BK, Wang Y, Hoffer BJ (2008). "Transgenic rodent models of Parkinson's disease". Acta Neurochir. Suppl. Acta Neurochirurgica Supplementum. 101: 89–92. ISBN 978-3-211-78204-0. PMC 2613245
 . PMID 18642640. doi:10.1007/978-3-211-78205-7_15.
. PMID 18642640. doi:10.1007/978-3-211-78205-7_15. - ↑ Blum, D; Torch, S; Lambeng, N; Nissou, M; Benabid, AL; Sadoul, R; Verna, JM (October 2001). "Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease". Progress in Neurobiology. 65 (2): 135–72. PMID 11403877. doi:10.1016/S0301-0082(01)00003-X.
- ↑ Feng, LR, Maguire-Zeiss KA (2010). "Gene Therapy in Parkinson's Disease: Rationale and Current Status". CNS Drugs. 24 (3): 177–92. PMC 2886503
 . PMID 20155994. doi:10.2165/11533740-000000000-00000.
. PMID 20155994. doi:10.2165/11533740-000000000-00000. - ↑ Lewitt PA, Rezai AR, Leehey MA (April 2011). "AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial". Lancet Neurol. 10 (4): 309–19. PMID 21419704. doi:10.1016/S1474-4422(11)70039-4.
- ↑ "Neurologix Files to Liquidate Under Chapter 7 Bankruptcy".
- ↑ "World's first Parkinson's vaccine is trialled". New Scientist. 7 June 2012.
- ↑ Redmond DE (October 2002). "Cellular replacement therapy for Parkinson's disease – where we are today?". The Neuroscientist. 8 (5): 457–88. PMID 12374430. doi:10.1177/107385802237703.
- ↑ "Stem Cell Research Aims to Tackle Parkinson's Disease". Retrieved 16 April 2010.
External links
| Classification |
V · T · D |
|---|---|
| External resources |
|
- Parkinson's Disease at DMOZ
- Parkinson's Disease: Hope Through Research (National Institute of Neurological Disorders and Stroke)
- World Parkinson Disease Association
- PDGENE – Database for Parkinson's Disease genetic association studies