Ovothiol A
 | |
| Names | |
|---|---|
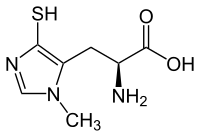
| IUPAC name
(2S)-2-Amino-3-(3-methyl-5-sulfanylimidazol-4-yl)propanoic acid | |
| Other names
1-N-Methyl-4-mercaptohistidine | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| MeSH | C061475 |
| PubChem CID |
|
| |
| |
| Properties | |
| C7H11N3O2S | |
| Molar mass | 201.24 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ovothiol A (N1-methyl-4-mercaptohistidine) is a highly reducing antioxidant mercaptohistidine, which accumulates to very high levels in the eggs of certain marine invertebrates, including sea urchins, scallops and starfish,[1] where it acts to scavenge hydrogen peroxide released during fertilization.[2]
This thiol is also found in some human pathogens including trypanosomes and members of the genus Leishmania.[3]
It is synthesized by the addition and oxidation of cysteine to histidine by 5-histidylcysteine sulfoxide synthase, followed by methylation and further reduction.
References
- ↑ Turner, E., Klevit, R., Hager, L. J. & Shapiro, B. M.; Klevit; Hager; Shapiro (1987). "Ovothiols, a family of redox-active mercaptohistidine compounds from marine invertebrate eggs". Biochemistry. 26 (13): 4028–4036. PMID 3651433. doi:10.1021/bi00387a043.
- ↑ Turner, E., Hager, L. J. & Shapiro, B. M.; Hager; Shapiro (1988). "Ovothiol replaces glutathione-peroxidase as a hydrogen-peroxide scavenger in sea-urchin eggs". Science. 242 (4880): 939–941. PMID 3187533. doi:10.1126/science.3187533.
- ↑ Spies, H.S. & Steenkamp, D.J. (1994). "Thiols of intracellular pathogens. Identification of ovothiol A in Leishmania donovani and structural analysis of a novel thiol from Mycobacterium bovis". Eur. J. Biochem. 224 (1): 203–213. PMID 8076641. doi:10.1111/j.1432-1033.1994.tb20013.x.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.