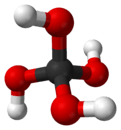
Orthocarbonic acid
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
orthocarbonic acid | |||
| Systematic IUPAC name
Methanetetrol[1] (substitutive) | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| ChemSpider | |||
| PubChem CID |
|||
| |||
| |||
| Properties | |||
| CH4O4 | |||
| Molar mass | 80.04 g·mol−1 | ||
| Related compounds | |||
| Related compounds |
Dihydroxymethylidene | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Orthocarbonic acid (methanetetrol) is the name given to a hypothetical compound with the chemical formula H4CO4 or C(OH)4. Its molecular structure consists of a single carbon atom bonded to four hydroxyl groups. It would be therefore a fourfold alcohol. In theory it could lose four protons to give the hypothetical oxocarbon anion CO4−
4 (orthocarbonate), and is therefore considered an oxoacid of carbon.
Orthocarbonic acid is highly unstable, decomposing spontaneously into carbonic acid monohydrate:[2][3]
- H4CO4 → H2CO3 + H2O.
Orthocarbonic acid is one of the group of carboxylic ortho acids that have the general structure of RC(OH)3.The term ortho acid is also used to refer to the most hydroxylated acid in a set of oxoacids. When drawn in two dimensions, the molecule resembles a swastika, and has therefore been called "Hitler's acid".[4]
Researchers at the Moscow Institute of Physics and Technology predict that orthocarbonic acid may occur in the interiors of Neptune and Uranus.[4]
Orthocarbonate anions
By loss of one through four protons, orthocarbonic acid could yield four anions: H
3CO−
4, H
2CO2−
4, HCO3−
4, and CO4−
4.
As of 2002, salts of these anions had yet to be observed. However, theoretical studies suggest that Na4CO4 might be stable.[5]
Orthocarbonate esters
The tetravalent moiety CO4 is found in stable organic compounds; they are formally esters of orthocarbonic acid, and therefore are called orthocarbonates. For example, ethyl orthocarbonate can be prepared by the reaction between chloropicrin and sodium ethoxide in ethanol.[6] Polyorthocarbonates are stable polymers that might have applications in absorbing organic solvents in waste treatment processes,[7] or in dental restorative materials.[8] The explosive trinitroethylorthocarbonate possesses a orthocarbonate core.
References
- ↑ "Methanetetrol - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ↑ Bohm S.; Antipova D.; Kuthan J. (1997). "A Study of Methanetetraol Dehydration to Carbonic Acid". International Journal of Quantum Chemistry. 62: 315–322. doi:10.1002/(SICI)1097-461X(1997)62:3<315::AID-QUA10>3.3.CO;2-N.
- ↑ Carboxylic Acids and Derivatives IUPAC Recommendations on Organic & Biochemical Nomenclature
- 1 2 Richard Gray (7 September 2016). What lies beneath Uranus and Neptune: Weird materials like 'Hitler's Acid' could lurk inside the planets. dailymail.co.uk
- ↑ Al-Shemali Musstafa; Boldyre Alexander I (2002). "Search for Ionic Orthocarbonates: Ab Initio Study of Na4CO4". J. Phys. Chem. A. 106 (38): 8951–8954. doi:10.1021/jp020207+.
- ↑ Orthocarbonic acid, tetraethyl ester Organic Syntheses, Coll. Vol. 4, p.457 (1963); Vol. 32, p.68 (1952)
- ↑ Sonmez, H.B.; Wudl, F. (2005). "Cross-linked poly(orthocarbonate)s as organic solvent sorbents". Macromolecules. 38 (5): 1623–1626. doi:10.1021/ma048731x.
- ↑ Stansbury, J.W. (1992). "Synthesis and evaluation of new oxaspiro monomers for double ring-opening polymerization". Journal of Dental Research. 71 (7): 1408–1412. PMID 1629456. doi:10.1177/00220345920710070901. Retrieved 2008-06-19.