Organophosphate

An organophosphate (sometimes abbreviated OP) or phosphate ester is the general name for esters of phosphoric acid. Organophosphates are the basis of many insecticides, herbicides, and nerve agents. The United States Environmental Protection Agency lists organophosphates as very highly acutely toxic to bees, wildlife, and humans.[1] Recent studies suggest a possible link to adverse effects in the neurobehavioral development of fetuses and children, even at very low levels of exposure. Organophosphates are widely used as solvents, plasticizers, and EP additives.
Organophosphates are widely employed both in natural and synthetic applications because of the ease with which organic groups can be linked together.
- OP(OH)3 + ROH → OP(OH)2(OR) + H2O
- OP(OH)2(OR) + R'OH → OP(OH)(OR)(OR') + H2O
- OP(OH)(OR)(OR') + R"OH → OP(OR)(OR')(OR") + H2O
The phosphate esters bearing OH groups are acidic and partially deprotonated in aqueous solution. For example, DNA and RNA are polymers of the type [PO2(OR)(OR')−]n. Polyphosphates also form esters; an important example of an ester of a polyphosphate is ATP, which is the monoester of triphosphoric acid (H5P3O10).
Alcohols can be detached from phosphate esters by hydrolysis, which is the reverse of the above reactions. For this reason, phosphate esters are common carriers of organic groups in biosynthesis.
Pesticides
The word "organophosphates", when appearing in communications (e.g., from the press or the government), in areas such as agriculture, the environment, and human and animal health, very often refers to a group of insecticides (pesticides) that act on the enzyme acetylcholinesterase (see also carbamates). Today, organophosphates make up about 50% of the killing agents in chemical pesticides.[2]
Organophosphate pesticides (OPPs), like some nerve agents, inhibit this neuromuscular enzyme, which is broadly essential for normal function in insects, but also in humans and many other animals.[3] OPPs affect this enzyme in varied ways, a principle one being through irreversible covalent inhibition,[4] and so create potentials for poisoning that vary in degree. The brain sends out neurotransmitters to the nerve endings in the body; organophosphates disrupt this process from occurring.This chemical, organophosphate works by disrupting the enzyme, acetylcholinesterase. Acetylcholinesterase break down the acetylcholine neurotransmitter, which sends out signals to other nerve endings in the body. Without these neurotransmitters from the brain, the nerves in the human body cannot function properly.[2]
For instance, parathion, one of the first OPPs commercialized, is many times more potent than malathion, an insecticide used in combating the Mediterranean fruit fly (Med-fly) and West Nile Virus-transmitting mosquitoes.[5] Human and animal exposure to them can be through ingestion of foods containing them, or via absorption through the skin or lungs.[3]
The human and animal toxicity of OPPs make them a societal health and environmental concern;[3] the EPA banned most residential uses of organophosphates in 2001, but their agricultural use, as pesticides on fruits and vegetables, is still permitted, and they are as is their use in mosquito abatement in public spaces such as parks.[3] For instance, the most commonly used OPP in the U.S., malathion,[6] sees wide application in agriculture, residential landscaping, and pest control programs (including mosquito control in public recreation areas).[7] As of 2010, forty such OPPs were registered for use in the U.S.,[8] with at least 73 million pounds used in one time period in agricultural and residential settings.[8] Commonly used organophosphates have included:
Studies have shown that prolonged exposure to OPPs—e.g., in the case of farm workers—can lead to health problems, including increased risks for cardiovascular and respiratory disease, and cancer. and in the case of pregnant women, exposure can result in premature births.[10] In addition, permanent damage to the brain’s chemical make-up, and changes in human behavior and emotion can occur to the fetus in pregnant women.[11]
Organophosphate pesticides degrade rapidly by hydrolysis on exposure to sunlight, air, and soil, although small amounts can be detected in food and drinking water. Their ability to degrade made them an attractive alternative to the persistent organochloride pesticides, such as DDT, aldrin, and dieldrin. Although organophosphates degrade faster than the organochlorides, the greater acute toxicity of OPPs result in the elevated risk associated with this class of compounds (see the Toxicity section below).
Nerve agents
History
Early pioneers in the field include Jean Louis Lassaigne (early 19th century) and Philippe de Clermont (1854). In 1932, German chemist Willy Lange and his graduate student, Gerde von Krueger, first described the cholinergic nervous system effects of organophosphates, noting a choking sensation and a dimming of vision after exposure. This discovery later inspired German chemist Gerhard Schrader at company IG Farben in the 1930s to experiment with these compounds as insecticides. Their potential use as chemical warfare agents soon became apparent, and the Nazi government put Schrader in charge of developing organophosphate (in the broader sense of the word) nerve gases. Schrader's laboratory discovered the G series of weapons, which included Sarin, Tabun, and Soman. The Nazis produced large quantities of these compounds, though did not use them during World War II. British scientists experimented with a cholinergic organophosphate of their own, called diisopropylfluorophosphate, during the war. The British later produced VX nerve agent, which was many times more potent than the G series, in the early 1950s, almost 20 years after the Germans had discovered the G series.
After World War II, American companies gained access to some information from Schrader's laboratory, and began synthesizing organophosphate pesticides in large quantities. Parathion was among the first marketed, followed by malathion and azinphosmethyl. The popularity of these insecticides increased after many of the organochlorine insecticides such as DDT, dieldrin, and heptachlor were banned in the 1970s.
Structural features
Effective organophosphates have the following structural features:
- A terminal oxygen connected to phosphorus by a double bond, i.e. a phosphoryl group
- Two lipophilic groups bonded to the phosphorus
- A leaving group bonded to the phosphorus, often a halide
Terminal oxygen vs. terminal sulfur
Thiophosphoryl compounds, those bearing the P=S functionality, are much less toxic than related phosphoryl derivatives. Thiophosphoryl compounds are not active inhibitors of acetylcholinesterase in either mammals or insects; in mammals, metabolism tends to remove lipophilic side groups from the phosphorus atom, while in insects it tends to oxidize the compound, thus removing the terminal sulfur and replacing it with a terminal oxygen, which allows the compound to more efficiently act as an acetylcholinesterase inhibitor.
Fine tuning
Within these requirements, a large number of different lipophilic and leaving groups have been used. The variation of these groups is one means of fine tuning the toxicity of the compound. A good example of this chemistry are the P-thiocyanate compounds which use an aryl (or alkyl) group and an alkylamino group as the lipophilic groups. The thiocyanate is the leaving group.
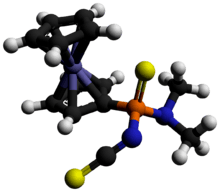
A German patent claimed that the reaction of 1,3,2,4-dithiadiphosphetane 2,4-disulfides with dialkyl cyanamides formed plant protection agents which contained six-membered (P-N=C-N=C-S-) rings. It has been proven in recent times by the reaction of diferrocenyl 1,3,2,4-dithiadiphosphetane 2,4-disulfide (and Lawesson's reagent) with dimethyl cyanamide that, in fact, a mixture of several different phosphorus-containing compounds is formed. Depending on the concentration of the dimethyl cyanamide in the reaction mixture, either a different six-membered ring compound (P-N=C-S-C=N-) or a nonheterocylic compound (FcP(S)(NR2)(NCS)) is formed as the major product; the other compound is formed as a minor product.
In addition, small traces of other compounds are also formed in the reaction. The ring compound (P-N=C-S-C=N-) {or its isomer} is unlikely to act as a plant protection agent, but (FcP(S)(NR2)(NCS)) compounds can act as nerve poisons in insects.
Health effects
Poisoning
Many "organophosphates" are potent nerve agents, functioning by inhibiting the action of acetylcholinesterase (AChE) in nerve cells. They are one of the most common causes of poisoning worldwide, and are frequently intentionally used in suicides in agricultural areas. Organophosphosphate pesticides can be absorbed by all routes, including inhalation, ingestion, and dermal absorption. Their inhibitory effects on the acetylcholinesterase enzyme lead to a pathological excess of acetylcholine in the body. Their toxicity is not limited to the acute phase, however, and chronic effects have long been noted. Neurotransmitters such as acetylcholine (which is affected by organophosphate pesticides) are profoundly important in the brain's development, and many organophosphates have neurotoxic effects on developing organisms, even from low levels of exposure. Other organophosphates are not toxic, yet their main metabolites, such as their oxons, are. Treatment includes both a pralidoxime binder and an anticholinergic such as atropine.
Chronic toxicity
Repeated or prolonged exposure to organophosphates may result in the same effects as acute exposure including the delayed symptoms. Other effects reported in workers repeatedly exposed include impaired memory and concentration, disorientation, severe depressions, irritability, confusion, headache, speech difficulties, delayed reaction times, nightmares, sleepwalking, drowsiness, or insomnia. An influenza-like condition with headache, nausea, weakness, loss of appetite, and malaise has also been reported.[12]
A recent study done by Madurai Kamaraj University in India have shown a direct correlation between usage of organophosphates and diabetes among Indian agricultural population.[13]
Low-level exposure
Even at relatively low levels, organophosphates may be hazardous to human health. The pesticides act on acetylcholinesterase,[14] an enzyme found in the brain chemicals closely related to those involved in ADHD, thus fetuses and young children, where brain development depends on a strict sequence of biological events, may be most at risk.[15] They can be absorbed through the lungs or skin or by eating them on food. According to a 2008 report from the U.S. Department of Agriculture, ″detectable″ traces of organophosphate were found in a representative sample of produce tested by the agency, 28% of frozen blueberries, 20% of celery, 27% of green beans, 17% of peaches, 8% of broccoli, and 25% of strawberries.[16]
The United States Environmental Protection Agency lists parathion as a possible human carcinogen.[17]
A 2007 study linked the organophosphate insecticide chlorpyrifos, which is used on some fruits and vegetables, with delays in learning rates, reduced physical coordination, and behavioral problems in children, especially ADHD.[18]
An organic diet is an effective way to reduce exposure to the organophosphorus pesticides commonly used in agricultural production.[19] Organophosphate metabolite levels rapidly drop, and for some metabolites, become undetectable in children's urine when an organic diet is consumed.[19] This is speculative based on a short study of 23 children, in which only a few organophosphate compounds were potentially reduced, no effect was shown for the majority of them that were found in the samples.
Cancer
The International Agency for Research on Cancer (IARC), found that organophosphates may possibly increased cancer risk.[20] Tetrachlorvinphos and parathion were classified as "possibly carcinogenic", malathion, diazinon, and glyphosate as “limited evidence of carcinogenity”.[20] The report concluded that glyphosate has the potential to cause DNA or chromosomal damage, and may cause non-Hodgkin lymphoma or cancer of lymph nodes.[20]
Affected populations
According to the EPA, organophosphate use in 2004 accounts for 40% of all insecticide products used in the United States.[21] Out of concerns for potential hazards of organophosphate exposure to child development, the EPA began phasing out forms of organophosphates used indoors in 2001.[21] However, organophosphates are by far the most commonly used pesticide in the agricultural industry in the form of glyphosate, the primary ingredient of Roundup, totaling to about 185 million pounds per year, as compared to the second most commonly used pesticide, atrazine, at 78 million pounds.[22] While it is used in forestry, urban, and home applications as well, the general population has been observed to have low exposure .[23] Thus, the primary affected population that faces exposure to organophosphates are farmworkers, especially those in countries that have fewer restrictions on its usage, such as in India.[24]
Farmworkers in the United States
In the United States, migrant and seasonal farmworkers are the most susceptible to organophosphate exposure. Of the U.S. farmworker population, there are about 4.2 million seasonal or migrant men, women, and even children, 70% of which are born in Mexico and an overwhelming majority of 90% of all are Latino.[25] This almost homogenous racial aspect of employment in farm work in the United States highly suggests social, economic, and political factors undercurrents that would explain their vulnerability.[26] Half of the farmworker population in the United States do not have legal documentation and two thirds live in poverty, making it difficult to fully understand and document the characteristics of this population with relative certainty.[27] Furthermore, the group faces linguistic barriers, with about 70% of the migrant seasonal farmworker population reporting that they cannot speak English well.[28]
In the United States, poverty and lack of documentation status puts migrant farmworkers in housing situations that make them far more likely to contract infectious or parasitic diseases and to suffer from chemical related ailments than the general U.S. population.[29] Field workers who are exposed to pesticides continue to further expose their families in their residences, especially through contaminated clothing in which the residue settles as house dust.[29]
Economic, social, racial, and political barriers make passing policy and creating protective measures less likely to occur; in the context of their jobs, migrant seasonal farm workers are structurally vulnerable to exploitation and working conditions that are Occupational Factors not up to health standards if they are unable to find the necessary physical and social resources to protect themselves.[30]
The nature of their job may require constant exposure to toxins and pesticides and subjects them to increasingly extreme weather as climate change progresses. Thus, migrant farm work has been ranked conservatively as possibly the second most dangerous jobs in the country.[31]
Proposal restrictions
According to the nongovernmental organisation Pesticide Action Network, parathion is one of the most dangerous pesticides.[32] In the US alone, more than 650 agricultural workers have been poisoned since 1966, of which 100 died. In underdeveloped countries, many more people have suffered fatal and nonfatal intoxications. The World Health Organization, PAN, and numerous environmental organisations propose a general and global ban. Its use is banned or restricted in 23 countries and its import is illegal in a total of 50 countries.[33] Its use was banned in the U.S. in 2000 and it has not been used since 2003.[33]
Other than for agricultural use, the organophosphate diazinon has been banned in the U.S. More than one million pounds of diazinon were used in California to control agricultural pests in 2000. The areas and crops on which diazinon are most heavily applied are structural pest control, almonds, head lettuce, leaf lettuce, and prunes.[34]
In May 2006, the Environmental Protection Agency (EPA) reviewed the use of dichlorvos and proposed its continued sale, despite concerns over its safety and considerable evidence suggesting it is carcinogenic and harmful to the brain and nervous system, especially in children. Environmentalists charge that the latest decision was the product of backroom deals with industry and political interference.[35]
In 2001, the EPA placed new restrictions on the use of the organophosphates phosmet and azinphos-methyl to increase protection of agricultural workers. The crop uses reported at that time as being phased out in four years included those for almonds, tart cherries, cotton, cranberries, peaches, pistachios, and walnuts. The crops with time-limited registration included apples/crab apples, blueberries, sweet cherries, pears, pine seed orchards, brussels sprouts, cane berries, and the use of azinphos-methyl by nurseries for quarantine requirements.[36] The labeled uses of phosmet include alfalfa, orchard crops (e.g. almonds, walnuts, apples, cherries), blueberries, citrus, grapes, ornamental trees (not for use in residential, park, or recreational areas) and nonbearing fruit trees, Christmas trees and conifers (tree farms), potatoes, and peas.[37] Azinphos-methyl has been banned in Europe since 2006.[38]
See also
- Activity based protein profiling using organophosphate-containing activity-based probes
- Pesticide toxicity to bees
- Toxic oil syndrome
References
- ↑ "Basic Information". Clothianidin – Registration Status and Related Information. U.S. EPA. 27 July 2012.
- 1 2 "Organophosphates: Background, Pathophysiology, Epidemiology". 2016-11-29.
- 1 2 3 4 Goodman, Brenda (21 Apr 2011). "Pesticide Exposure in Womb Linked to Lower IQ". Health & Pregnancy. WebMD.
- ↑ Peter, J. V.; Sudarsan, T. I.; Moran, J. L. (2014). "Clinical features of organophosphate poisoning: A review of different classification systems and approaches". Indian Journal of Critical Care Medicine. 18 (11): 735–745. PMC 4238091
 . doi:10.4103/0972-5229.144017.
. doi:10.4103/0972-5229.144017. - ↑ "Malathion". Environmental Protection Agency.
- 1 2 Bonner MR, Coble J, Blair A, et al. (2007). "Malathion Exposure and the Incidence of Cancer in the Agricultural Health Study". American Journal of Epidemiology. 166 (9): 1023–34. PMID 17720683. doi:10.1093/aje/kwm182.
- 1 2 Malathion for Mosquito Control
- 1 2 Maugh II, Thomas H. (16 May 2010). "Study links pesticide to ADHD in children". Los Angeles Times.
- ↑ "Fenitrothion". Pesticide Information Profiles. Extension Toxicology Network. Sep 1995.
- ↑ Morello-Frosch, Rachel; Zuk, Miriam; Jerrett, Michael; Shamasunder, Bhavna; Kyle, Amy D. (May 2011). "Understanding the Cumulative Impacts of Inequalities in Environmental Health: Implications for Policy". Health Affairs. 30 (5): 881. doi:10.1377/hlthaff.2011.0153.
- ↑ "Organophosphates". tools.niehs.nih.gov. Retrieved 2017-04-24.
- ↑ "PARATHION". Pesticide Information Profiles. Extension Toxicology Network. Sep 1993.
- ↑ Velmurugan, Ganesan; Ramprasath, Tharmarajan; Swaminathan, Krishnan; Mithieux, Gilles; Rajendhran, Jeyaprakash; Dhivakar, Mani; Parthasarathy, Ayothi; Babu, D.D. Venkatesh; Thumburaj, Leishman John; Freddy, Allen J.; Dinakaran, Vasudevan; Puhari, Shanavas Syed Mohamed; Rekha, Balakrishnan; Christy, Yacob Jenifer; Anusha, Sivakumar; Divya, Ganesan; Suganya, Kannan; Meganathan, Boominathan; Kalyanaraman, Narayanan; Vasudevan, Varadaraj; Kamaraj, Raju; Karthik, Maruthan; Jeyakumar, Balakrishnan; Abhishek, Albert; Paul, Eldho; Pushpanathan, Muthuirulan; Rajmohan, Rajamani Koushick; Velayutham, Kumaravel; Lyon, Alexander R.; Ramasamy, Subbiah (2017). "Gut microbial degradation of organophosphate insecticides-induces glucose intolerance via gluconeogenesis". Genome Biology. 18. doi:10.1186/s13059-016-1134-6.
- ↑ "Organophosphates FAQs". Centers for Disease Control and Prevention. DHHS Department of Health and Human Services. Retrieved 6 February 2016.
- ↑ Jurewicz, Joanna; Hanke, Wojciech (9 Jul 2008). "Prenatal and Childhood Exposure to Pesticides and Neurobehavioral Development: Review of Epidemiological Studies". International Journal of Occupational Medicine and Environmental Health. Versita, Warsaw. 21 (2): 121–132. ISSN 1896-494X. PMID 18614459. doi:10.2478/v10001-008-0014-z.
- ↑ "Study: ADHD linked to pesticide exposure". CNN. 17 May 2010.
- ↑ "Parathion (CASRN 56-38-2)". IRIS Summaries. U.S. EPA. 9 Aug 2012.
- ↑ Study Links Organophosphate Insecticide Used on Corn With ADHD. Beyond Pesticides. 5 January 2007.
- 1 2 Lu, Chensheng; Toepel, Kathryn; Irish, Rene; Fenske, Richard A.; Barr, Dana B.; Bravo, Roberto (2006). "Organic Diets Significantly Lower Children's Dietary Exposure to Organophosphorus Pesticides". Environmental Health Perspectives. 114 (2): 260–3. PMC 1367841
 . PMID 16451864. doi:10.1289/ehp.8418.
. PMID 16451864. doi:10.1289/ehp.8418. - 1 2 3 "IARC Monographs Volume 112: evaluation of five organophosphate insecticides and herbicides" (PDF). World Health Organization.
- 1 2 "Environmental Health Perspectives – Population-Based Biomonitoring of Exposure to Organophosphate and Pyrethroid Pesticides in New York City". ehp.niehs.nih.gov. Retrieved 2017-04-24.
- ↑ Fishel, Frederick M. “Pesticide Use Trends in the United States: Agricultural Pesticides.” Jan. 2007
- ↑ IARC Monographs Volume 112: evaluation of five organophosphate insecticides and herbicides
- ↑ "Organophosphates: A Common But Deadly Pesticide". 2013-07-18. Retrieved 2017-04-24.
- ↑ Arcury, T A; Quandt, S A; Dearry, A (2017-04-24). "Farmworker pesticide exposure and community-based participatory research: rationale and practical applications.". Environmental Health Perspectives. 109 (Suppl 3): 429–434. ISSN 0091-6765. PMC 1240561
 . PMID 11427392.
. PMID 11427392. - ↑ Joan D. Flocks, e Environmental and Social Injustice of Farmworker Pesticide Exposure, 19 Geo. J. on Poverty L. & Pol'y 255 (2012), available at http://scholarship.law.u.edu/facultypub/268
- ↑ Villarejo, Don (2003-11-28). "The Health of U.S. Hired Farm Workers". doi:10.1146/annurev.publhealth.24.100901.140901. Retrieved 2017-04-24.
- ↑ Farmworker Health Factsheet. N.p.: National Center For Farmworker Health, Aug. 2012. PDF. http://www.ncfh.org/uploads/3/8/6/8/38685499/fs-facts_about_farmworkers.pdf
- 1 2 Hansen, Eric; Donohoe, Martin (2003-05-01). "Health issues of migrant and seasonal farmworkers". Journal of Health Care for the Poor and Underserved. 14 (2): 153–164. ISSN 1049-2089. PMID 12739296.
- ↑ Quesada, James; Hart, Laurie K.; Bourgois, Philippe (2017-04-24). "Structural Vulnerability and Health: Latino Migrant Laborers in the United States". Medical anthropology. 30 (4): 339–362. ISSN 0145-9740. PMC 3146033
 . PMID 21777121. doi:10.1080/01459740.2011.576725.
. PMID 21777121. doi:10.1080/01459740.2011.576725. - ↑ "NOW with Bill Moyers. Politics & Economy. On the Border. Migrant Labor in United States | PBS". www.pbs.org. Retrieved 2017-04-24.
- ↑ S. Kegley; B. Hill; S. Orme. "Parathion - Identification, toxicity, use, water pollution potential, ecological toxicity and regulatory information". Pesticide Action Network.
- 1 2 "Parathion (Ethyl) - Remaining Use Canceled 9/00". Pesticide Active Ingredient Information. Cornell University. 13 Oct 2000.
- ↑ "Diazinon". Agrochemicals. Great Vista Chemicals.
- ↑ Raeburn, Paul (14 Aug 2006). "Slow-Acting". Scientific American.
- ↑ Hess, Glenn (1 Nov 2011). "US EPA restricts pesticides azinphos-methyl, phosmet". ICIS.com.
- ↑ Peck, Chuck; Aubee, Catherine (29 Mar 2010). "Risks of Phosmet Use to the Federally Threatened and Endangered California Tiger Salamander (Ambystoma californiense)" (PDF). Pesticide Effects Determinations. Environmental Fate and Effects Division, Office of Pesticide Programs.
- ↑ Scott, Alex (4 Aug 2008). "Europe Rejects Appeal for Use of Azinphos-methyl Pesticide". IHS Chemical Week.
External links
- Costa LG (April 2006). "Current issues in organophosphate toxicology". Clin. Chim. Acta. 366 (1–2): 1–13. PMID 16337171. doi:10.1016/j.cca.2005.10.008..
- ATSDR Case Studies in Environmental Medicine: Cholinesterase Inhibitors, Including Pesticides and Chemical Warfare Nerve Agents U.S. Department of Health and Human Services
- Organophosphates - an article by Frances M Dyro, MD
- NYTimes: E.P.A. Recommends Limits On Thousands of Pesticides
- EPA OP pesticide site
- Epidemiological study of the relationships between exposure to organophosphate pesticides and indices of chronic peripheral neuropathy, and neuropsychological abnormalities in sheep farmers and dippers. Phase 3 Report Institute of Occupational Medicine TM/99/02c
- Epidemiological study of the relationships between exposure to organophosphate pesticides and indices of chronic peripheral neuropathy, and neuropsychological abnormalities in sheep farmers and dippers. Phase 2 Report Institute of Occupational Medicine TM/99/0b
- Epidemiological study of the relationships between exposure to organophosphate pesticides and indices of chronic peripheral neuropathy, and neuropsychological abnormalities in sheep farmers and dippers. Phase 1 Report Institute of Occupational Medicine TM/99/0a