Oncolytic adenovirus
Adenovirus varieties have been explored extensively as a viral vector for gene therapy and also as an oncolytic virus.[1][2]
Of the many different viruses being explored for oncolytic potential, an adenovirus was the first to be approved by a regulatory agency, the genetically modified H101 strain. It gained regulatory approval in 2005 from China's State Food and Drug Administration (SFDA) for the treatment of head and neck cancer.[3][4]
Engineering of oncolytic adenovirus
Adenoviruses have so far been through three generations of development.[5] Some of the strategies for modification of adenoviruses are described below.
Attenuation
For adenovirus replication to occur, the host cell must be induced into S phase by viral proteins interfering with cell cycle proteins. The adenoviral E1A gene is responsible for inactivation of several proteins, including retinoblastoma, allowing entry into S-phase. The adenovirus E1B55kDa gene cooperates with another adenoviral product, E4ORF6, to inactivate p53, thus preventing apoptosis. It was initially proposed that an adenovirus mutant lacking the E1B55kDa gene, dl1520 (ONYX-015), could replicate selectively in p53 deficient cells.
A conditionally replicative adenovirus (CRAd) with a 24 base pair deletion in the retinoblastoma-binding domain of the E1A protein (Ad5- Δ24E3), is unable to silence retinoblastoma, and therefore unable to induce S-phase in host cells.[6] This restricts Ad5-Δ24E3 to replication only in proliferating cells, such as tumour cells.
Targeting
The most commonly used group of adenoviruses is serotype 5 (Ad5), whose binding to host cells is initiated by interactions between the cellular coxsackie virus and adenovirus receptor (CAR), and the knob domain of the adenovirus coat protein trimer. CAR is necessary for adenovirus infection.[7] Although expressed widely in epithelial cells, CAR expression in tumours is extremely variable, leading to resistance to Ad5 infection.[7] Retargeting of Ad5 from CAR, to another receptor that is ubiquitously expressed on cancer cells, may overcome this resistance.[7]
- Adapter molecules
- Bi-specific adapter molecules can be administered along with the virus to redirect viral coat protein tropism. These molecules are fusion proteins that are made up of an antibody raised against the knob domain of the adenovirus coat protein, fused to a natural ligand for a cell-surface receptor.[8] The use of adapter molecules has been shown to increase viral transduction. However, adapters add complexity to the system, and the effect of adapter molecule binding on the stability of the virus is uncertain.
- Coat-protein modification
- This method involves genetically modifying the fiber knob domain of the viral coat protein to alter its specificity. Short peptides added to the C-terminal end of the coat protein successfully altered viral tropism.[9] The addition of larger peptides to the C-terminus is not viable because it reduces adenovirus integrity, possibly due to an effect on fiber trimerisation. The fiber protein also contains an HI-loop structure, which can tolerate peptide insertions of up to 100 residues without any negative effects on adenovirus integrity. An RGD motif inserted into the HI loop of the fiber knob protein, shifts specificity toward integrins, which are frequently over-expressed in oesophageal adenocarcinoma.[9][10] When combined with a form of non-transductional targeting, these viruses proved to be effective and selective therapeutic agents for Oesophageal Adenocarcinoma.
- Transcriptional targeting
- This approach takes advantage of deregulated promoter to drive and control the expression of adenoviral genes. For instance, Cyclooxygenase-2 enzyme (Cox-2) expression is elevated in a range of cancers, and has low liver expression, making it a suitable tumour-specific promoter. AdCox2Lluc is a CRAd targeted against oesophageal adenocarcinoma by placing the early genes under the control of a Cox-2 promoter (adenoviruses have two early genes, E1A and E1B, that are essential for replication).[10] When combined with transductional targeting, AdCox2Lluc showed potential for treatment of Oesophageal Adenocarcinoma. Cox-2 is also a possible tumour-specific promoter candidate for other cancer types, including ovarian cancer.
- A suitable tumour-specific promoter for prostate cancer is prostate-specific antigen (PSA), whose expression is greatly elevated in prostate cancer. CN706 is a CRAd with a PSA tumour-specific promoter driving expression of the adenoviral E1A gene, required for viral replication. The CN706 titre is significantly greater in PSA-positive cells.[11]
- Post-Transcriptional detargeting
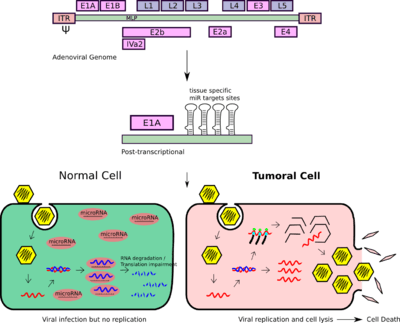
- Another layer of regulation that has emerged to control adenoviral replication is the use of microRNAs (miRNA) artificial target sites or miRNA response elements (MREs). Differential expression of miRNAs between healthy tissues and tumors permit to engineer oncolytic viruses in order to have their ability to replicate impaired in those tissues of interest while allowing its replication in the tumor cells.
| Tissue/cell-type | Enriched miRNA | Use of the MRE | References |
|---|---|---|---|
| Liver | miR-122 | Prevent liver toxicity, hepatotoxicity | [12] |
| Muscle | miR-133, miR-206 | Prevent muscle inflammation, myositis | [13] |
| Pancreas | miR-148a | Promote pancreatic tumor targeting | [14] |
| Prostate | miR-143, miR-145 | Promote prostate tumor targeting | [15] |
| Neuron | miR-124 | Promote astrocyte targeting | [16] |
Examples
Oncorine (H101)
H101 and the very similar Onyx-015 have been engineered to remove a viral defense mechanism that interacts with a normal human gene p53, which is very frequently dysregulated in cancer cells.[4] Despite the promises of early in vivo lab work, these viruses do not specifically infect cancer cells, but they still kill cancer cells preferentially.[4] While overall survival rates are not known, short-term response rates are approximately doubled for H101 plus chemotherapy when compared to chemotherapy alone.[4] It appears to work best when injected directly into a tumour, and when any resulting fever is not suppressed.[4] Systemic therapy (such as through infusion through an intravenous line) is desirable for treating metastatic disease.[17] It is now marketed under the brand name Oncorine.[18]
Onyx-015 (dl1520)
Onyx-015 (originally named Ad2/5 dl1520[19][20]) is an experimental oncolytic virus created by genetically engineering an adenovirus.[19][21] It has been trialed as a possible treatment for cancer. The E1B-55kDa gene has been deleted allowing the virus to selectively replicate in and lyse p53-deficient cancer cells.[22]
Directed Evolution
Traditional research has focussed on species C Adenovirus serotype 5 (Ad5) for creating oncolytic vaccines for the potential use as cancer treatment. However, recent data suggests that it may not be the best virus serotype for deriving all oncolytic agents for treating human malignancies.[23] For example, oncolytic vaccines based on the Ad5 serotype have relatively poor clinical efficacy as monotherapies.[24][25][26][27] The need for increased potency (infectivity and lytic activity) has led to an expanded search involving a larger number of less well studied adenovirus serotypes.
ColoAd1
One non-species C oncolytic adenovirus currently in development is ColoAd1. It was created using a process of “directed evolution”. This involves the creation of new viral variants or serotypes specifically directed against tumour cells via rounds of directed selection using large populations of randomly generated recombinant precursor viruses. The increased biodiversity produced by the initial homologous recombination step provides a large random pool of viral candidates which can then be passed through a series of selection steps designed to lead towards a pre-specified outcome (e.g. higher tumor specific activity) without requiring any previous knowledge of the resultant viral mechanisms that are responsible for that outcome.[28] One particular application of this approach produced ColoAd1, which is a novel Ad11p/Ad3 chimeric Group B oncolytic virus with specificity for human colon cancer and a broad spectrum of anti-cancer activity in common solid tumours.[28] The therapeutic efficacy of ColoAd1 is currently being evaluated in three ongoing clinical trials (see the EU Clinical Trials Register for further details). ColoAd1 potency can be further enhanced via the use of therapeutic transgenes, which can be introduced into the ColoAd1 genome without compromising the selectivity or activity of the virus. Recent studies with ColoAd1 have shown a unique mechanism of cell death similar to Oncosis with expression of inflammatory cell death markers and cell membrane blistering.[29]
Background
Tumours form in cells when mutations in genes involved in cell cycle control and apoptosis accumulate over time.[30] Most tumours studied, have defects in the p53 tumor suppressor pathway.[31] p53 is a transcription factor that plays a role in apoptosis, cell cycle and DNA repair. It blocks cell progression in response to cellular stress or DNA damage. Many viruses replicate by altering the cell cycle and exploiting the same pathways that are altered in cancer cells.[32] E1B proteins produced by adenoviruses protect the infected cell by binding to and degrading the p53 transcription factors,[33] preventing it from targeting the cell for apoptosis. This allows the virus to replicate, package its genome, lyse the cell and spread to new cells.
This gave rise to the idea that an altered adenovirus could be used to target and eliminate cancer cells. Onyx-015 is an adenovirus that was developed in 1987 with the function of the E1B gene knocked out,[34] meaning cells infected with Onyx-015 are incapable of blocking p53's function. If Onyx-015 infects a normal cell, with a functioning p53 gene, it will be prevented from multiplying by the action of the p53 transcription factor. However, if Onyx-015 infects a p53 deficient cell it should be able to survive and replicate, resulting in selective destruction of cancer cells.
Clinical trials
ColoAd1 from PsiOxus Therapeutics has entered Phase I/II clinical study with its oncolytic vaccine. Phase I of the trial recruited patients with metastatic solid tumors and showed evidence for virus replication within tumour sites after intravenous delivery. The second phase of the ColoAd1 study will involve the comparison of intra-tumoural versus intravenous injection to examine viral replication, viral spread, tumour necrosis and anti-tumoural immune responses (see the EU Clinical Trials Register for further details).
ONYX-015 (dl1520)/H101
Patents for the therapeutic use of ONYX-015 are held by ONYX Pharmaceuticals[35][36] and it was used in combination with the standard chemotherapeutic agents cisplatin and 5-fluorouracil to combat head and neck tumours.[37] Onyx-015 has been extensively tested in clinical trials, with the data indicating that it is safe and selective for cancer.[38] However, limited therapeutic effect has been demonstrated following injection and systemic spread of the virus was not detected.[39] ONYX-015 when combined with chemotherapy, however, proved reasonably effective in a proportion of cases. During these trials a plethora of reports emerged challenging the underlying p53-selectivity, with some reports showing that in some cancers with a wild-type p53 ONYX-015 actually did better than in their mutant p53 counterparts. These reports slowed the advancement through Phase III trials in the US, however recently China licensed ONYX-015 for therapeutic use as H101.[40] Further development of Onyx-015 was abandoned in the early 2000s, the exclusive rights being licensed to the Chinese company, Shanghai Sunway Biotech. On November 17, 2005, the Chinese State Food and Drug Administration approved H101, an oncolytic adenovirus similar to Onyx-015 (E1B-55K/E3B-deleted), for use in combination with chemotherapy for the treatment of late-stage refractory nasopharyngeal cancer.[41][42] Outside of China, the push to the clinic for ONYX-015 has been largely been discontinued for financial reasons and until a real mechanism can be found.[43]
See also
References
- ↑ Pandha, K. J. Harrington ; edited by Richard G. Vile, Hardev (2008). Viral therapy of cancer. Hoboken, N.J.: Wiley. pp. 1–13. ISBN 9780470019221.
- ↑ Fillat, Cristina (2010). "Controlling Adenoviral Replication to Induce Oncolytic Efficacy" (PDF). The Open Gene Therapy Journal. 3: 15–23. doi:10.2174/1875037001003010015.
- ↑ Frew, Sarah E; Sammut, Stephen M; Shore, Alysha F; Ramjist, Joshua K; Al-Bader, Sara; Rezaie, Rahim; Daar, Abdallah S; Singer, Peter A (2008). "Chinese health biotech and the billion-patient market". Nature Biotechnology. 26 (1): 37–53. PMID 18183014. doi:10.1038/nbt0108-37.
- 1 2 3 4 5 Garber, K. (2006). "China Approves World's First Oncolytic Virus Therapy for Cancer Treatment". JNCI Journal of the National Cancer Institute. 98 (5): 298–300. PMID 16507823. doi:10.1093/jnci/djj111.
- ↑ Doronin, K; Shayakhmetov, DM (2012). "Construction of targeted and armed oncolytic adenoviruses.". Methods in molecular biology (Clifton, N.J.). 797: 35–52. PMID 21948467. doi:10.1007/978-1-61779-340-0_3.
- ↑ Carette, J. E.; Overmeer, RM; Schagen, FH; Alemany, R; Barski, OA; Gerritsen, WR; Van Beusechem, VW (2004). "Conditionally Replicating Adenoviruses Expressing Short Hairpin RNAs Silence the Expression of a Target Gene in Cancer Cells". Cancer Research. 64 (8): 2663–7. PMID 15087375. doi:10.1158/0008-5472.CAN-03-3530.
- 1 2 3 Li, Y; Pong, RC; Bergelson, JM; Hall, MC; Sagalowsky, AI; Tseng, CP; Wang, Z; Hsieh, JT (1999). "Loss of adenoviral receptor expression in human bladder cancer cells: A potential impact on the efficacy of gene therapy". Cancer Research. 59 (2): 325–30. PMID 9927041.
- ↑ Everts, M; Curiel, DT (September 2004). "Transductional targeting of adenoviral cancer gene therapy.". Current gene therapy. 4 (3): 337–46. PMID 15384947. doi:10.2174/1566523043346372.
- 1 2 Wickham, Thomas J. (2003). "Ligand-directed targeting of genes to the site of disease". Nature Medicine. 9 (1): 135–9. PMID 12514727. doi:10.1038/nm0103-135.
- 1 2 Davydova, J.; Le, LP; Gavrikova, T; Wang, M; Krasnykh, V; Yamamoto, M (2004). "Infectivity-Enhanced Cyclooxygenase-2-Based Conditionally Replicative Adenoviruses for Esophageal Adenocarcinoma Treatment". Cancer Research. 64 (12): 4319–27. PMID 15205347. doi:10.1158/0008-5472.CAN-04-0064.
- ↑ Rodriguez, R; Schuur, ER; Lim, HY; Henderson, GA; Simons, JW; Henderson, DR (1997). "Prostate attenuated replication competent adenovirus (ARCA) CN706: A selective cytotoxic for prostate-specific antigen-positive prostate cancer cells". Cancer Research. 57 (13): 2559–63. PMID 9205053.
- ↑ Ylösmäki, E (2008). "Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNA". Journal of Virology. 82 (22): 11009–11015. PMC 2573287
 . PMID 18799589. doi:10.1128/JVI.01608-08.
. PMID 18799589. doi:10.1128/JVI.01608-08. - ↑ Kelly, EJ (2008). "Engineering microRNA responsiveness to decrease virus pathogenicity.". Nature Medicine. 14 (11): 1278–1283. PMID 18953352. doi:10.1038/nm.1776.
- ↑ Bofill-De Ros, X (2014). "MiR-148a- and miR-216a-regulated Oncolytic Adenoviruses Targeting Pancreatic Tumors Attenuate Tissue Damage Without Perturbation of miRNA Activity". Molecular Therapy. 22 (9): 1665–1677. PMC 4435498
 . PMID 24895996. doi:10.1038/mt.2014.98.
. PMID 24895996. doi:10.1038/mt.2014.98. - ↑ Lee, CY (2009). "MicroRNA regulation of oncolytic herpes simplex virus-1 for selective killing of prostate cancer cells" (PDF). Clinical Cancer Research. 15 (16): 5126–5135. PMID 19671871. doi:10.1158/1078-0432.ccr-09-0051.
- ↑ Colin, A (2009). "Engineered lentiviral vector targeting astrocytes in vivo". Glia. 57 (6): 667–679. PMID 18942755. doi:10.1002/glia.20795.
- ↑ Ayllón Barbellido, S; Campo Trapero, J; Cano Sánchez, J; Perea García, MA; Escudero Castaño, N; Bascones Martínez, A (2008). "Gene therapy in the management of oral cancer: Review of the literature" (PDF). Medicina oral, patologia oral y cirugia bucal. 13 (1): E15–21. PMID 18167474.
- ↑ Guo, J; Xin, H (Nov 24, 2006). "Chinese gene therapy. Splicing out the West?". Science. 314 (5803): 1232–5. PMID 17124300. doi:10.1126/science.314.5803.1232.
- 1 2 Barker, Douglas D.; Berk, Arnold J. (1987). "Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection". Virology. 156 (1): 107–121. PMID 2949421. doi:10.1016/0042-6822(87)90441-7.
- ↑ Heise, Carla; Sampson-Johannes, Adam; Williams, Angelica; Mccormick, Frank; Von Hoff, Daniel D.; Kirn, David H. (June 1997). "ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents". Nature Medicine. 3 (6): 639–645. doi:10.1038/nm0697-639.
- ↑ Definition of ONYX-015 - National Cancer Institute Drug Dictionary
- ↑ John Nemunaitis; Ian Ganly; Fadlo Khuri; James Arseneau; Joseph Kuhn; Todd McCarty; Stephen Landers; Phillip Maples; Larry Rome; Britta Randlev; Tony Reid; Sam Kaye; David Kirn (2000). "Selective Replication and Oncolysis in p53 Mutant Tumors with ONYX-015, an E1B-55kD Gene-deleted Adenovirus, in Patients with Advanced Head and Neck Cancer: A Phase II Trial". Cancer Res. 60 (22): 6359–66. PMID 11103798.
- ↑ Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours" Nat Rev Cancer 2005;5:965–976.
- ↑ Kirn D (2001). "Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015) results of phase I and II trials". Expert Opin Biol Ther. 1: 525–538. doi:10.1517/14712598.1.3.525.
- ↑ Yu DC, Working P, Ando D (2002). "Selectively replicating oncolytic adenoviruses as cancer therapeutics". Curr Opin Mol Ther. 4: 435–443.
- ↑ Reid T, Warren R, Kirn D (2002). "Intravascular adenoviral agents in cancer patients: lessons from clinical trials". Cancer Gene Ther. 9: 979–986. doi:10.1038/sj.cgt.7700539.
- ↑ Freytag SO, Khil M, Stricker H, Peabody J, Menon M, et al. (2002). "Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer". Cancer Res. 62: 4968–4976.
- 1 2 Kuhn I, Harden P, Bauzon M, Chartier C, Nye J, Thorne S, Reid T, Ni S, Lieber A, Fisher K, Seymour L, Rubanyi GM, Harkins RN, Hermiston TW (2008). "Directed evolution generates a novel oncolytic virus for the treatment of colon cancer". PLoS ONE. 3 (6): e2409. PMC 2423470
 . PMID 18560559. doi:10.1371/journal.pone.0002409.
. PMID 18560559. doi:10.1371/journal.pone.0002409. - ↑ Dyer A, Di Y, Calderon H, Illingworth S, Kueberuwa G, Tedcastle A, Jakeman P, Chia SL, Brown A, Silva M, Barlow D, Beadle J, Hermiston T, Ferguson D, Champion B, Fisher K, Seymour L (2017). "Oncolytic Group B Adenovirus Enadenotucirev Mediates Non-apoptotic Cell Death with Membrane Disruption and Release of Inflammatory Mediators". Molecular Therapy Oncolytics. 4: 18–30. doi:10.1016/j.omto.2016.11.003.
- ↑ Vogelstein, B.; Kinzler, K. (1993). "The multistep nature of cancer". Trends in Genetics. 9 (4): 138–141. PMID 8516849. doi:10.1016/0168-9525(93)90209-Z.
- ↑ Levine, A. (1997). "P53, the Cellular Gatekeeper for Growth and Division". Cell. 88 (3): 323–331. PMID 9039259. doi:10.1016/S0092-8674(00)81871-1.
- ↑ Ries, S.; Korn, W. (2002). "ONYX-015: mechanisms of action and clinical potential of a replication-selective adenovirus". British Journal of Cancer. 86 (1): 5–11. PMC 2746528
 . PMID 11857003. doi:10.1038/sj.bjc.6600006.
. PMID 11857003. doi:10.1038/sj.bjc.6600006. - ↑ Yew, P.; Berk, A. (1992). "Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein". Nature. 357 (6373): 82–85. Bibcode:1992Natur.357...82Y. PMID 1533443. doi:10.1038/357082a0.
- ↑ Barker DD, Berk AJ (1987). "Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection". Virology. 156 (1): 107–121. PMID 2949421. doi:10.1016/0042-6822(87)90441-7.
- ↑ Bischoff, J. R.; Kirn, D. H.; Williams, A.; Heise, C.; Horn, S.; Muna, M.; Ng, L.; Nye, J. A.; Sampson-Johannes, A.; Fattaey, A.; McCormick, F. (1996). "An Adenovirus Mutant That Replicates Selectively in p53- Deficient Human Tumor Cells". Science. 274 (5286): 373–376. PMID 8832876. doi:10.1126/science.274.5286.373.
- ↑ US patent 5677178, McCormick; Francis, "Cytopathic viruses for therapy and prophylaxis of neoplasia", issued 1997-10-14
- ↑ Khuri, F.; Nemunaitis, J.; Ganly, I.; Arseneau, J.; Tannock, I.; Romel, L.; Gore, M.; Ironside, J.; MacDougall, R.; Heise, C.; Randlev, B.; Gillenwater, A. M.; Bruso, P.; Kaye, S. B.; Hong, W. K.; Kirn, D. H. (2000). "A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer". Nature Medicine. 6 (8): 879–885. PMID 10932224. doi:10.1038/78638.
- ↑ Kirn, D.; Thorne, S. (2009). "Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer". Nature Reviews. Cancer. 9 (1): 64–71. PMID 19104515. doi:10.1038/nrc2545.
- ↑ Liu, T.; Hwang, T.; Bell, J.; Kirn, D. (2008). "Translation of targeted oncolytic virotherapeutics from the lab into the clinic, and back again: a high-value iterative loop". Molecular Therapy. 16 (6): 1006–1008. PMID 18500240. doi:10.1038/mt.2008.70.
- ↑ Moon Crompton, Anne; Kirn, David H. (2007). "From ONYX-015 to Armed Vaccinia Viruses: The Education and Evolution of Oncolytic Virus Development". Current Cancer Drug Targets. 7 (2): 133–9. PMID 17346104. doi:10.2174/156800907780058862.
- ↑ Liu, T.; Kirn, D. (2008). "Gene therapy progress and prospects cancer: oncolytic viruses". Gene therapy. 15 (12): 877–884. PMID 18418413. doi:10.1038/gt.2008.72.
- ↑ Chinese State FDA approval
- ↑ "Onyx Increases Development Focus on Bay 43-9006" (Press release). Onyx Pharma. 27 February 2003. Retrieved 25 July 2006.