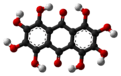
Octahydroxyanthraquinone
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,2,3,4,5,6,7,8-Octahydroxy-9,10-anthracenedione | |||
| Other names
Octahydroxyanthracenedione | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| PubChem CID |
|||
| |||
| |||
| Properties | |||
| C14H8O12 | |||
| Molar mass | 368.21 g·mol−1 | ||
| log P | -0.291 | ||
| Acidity (pKa) | 5.358 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Octahydroxyanthraquinone is an organic compound with formula C
14H
8O
12, formally derived from anthraquinone by replacement of 8 hydrogen atoms by hydroxyl groups.
The compound was obtained in 1911 by Georg von Georgievics[1][2] and can be obtained through oxidation of rufigallol (1,2,3,5,6,7-hexahydroxyanthraquinone) with boric acid and mercuric oxide in sulfuric acid at 250 °C (482 °F).[3]
Esters of octahydroxyanthraquinone, where all eight hydroxyls are replaced by straight-chain 1-alkanecarboxylate groups H
3C-(CH
2)n-COO-, with n between 6 and 14, are liquid crystals and have been studied for possible LCD applications.[3]
Octahydroxyanthraquinone is active against the malaria parasite, but rufigallol (1,2,3,5,6,7-hexahydroxyanthraquinone) is 22 times more potent.[4]
References
- ↑ Georgievics, G. v. (1911). "Darstellung und Eigenschaften des Octooxyanthrachinons". Monatshefte für Chemie. 32: 347. doi:10.1007/BF01518160.
- ↑ Wahl, Andre; Atack, F. W (1919) The Manufacture Of Organic Dyestuffs. G. Bell And Sons, Limited. Online version accessed on 2010-01-22.
- 1 2 Kumar, Sandeep (2008). "Rufigallol-based self-assembled supramolecular architectures". Phase Transitions. 81: 113. doi:10.1080/01411590701601610.
- ↑ Winter, R (1995). "Hydroxy-anthraquinones as antimalarial agents". Bioorganic & Medicinal Chemistry Letters. 5: 1927. doi:10.1016/0960-894X(95)00326-O.