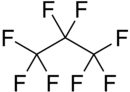
Octafluoropropane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Octafluoropropane | |||
| Other names
Freon 218 Perfluoropropane RC 218, PFC 218 R218 Flutec PP30 genetron 218 | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.857 | ||
| KEGG | |||
| PubChem CID |
|||
| RTECS number | TZ5255000 | ||
| UNII | |||
| |||
| |||
| Properties | |||
| C3F8 | |||
| Molar mass | 188.02 g/mol | ||
| Appearance | Colorless gas with faintly sweet odor | ||
| Density | 8.17 g/l, gas | ||
| Melting point | −183 °C (−297.4 °F; 90.1 K) | ||
| Boiling point | −36.7 °C (−34.1 °F; 236.5 K) | ||
| Structure | |||
| 0.014 D | |||
| Hazards | |||
| Main hazards | Suffocation | ||
| Safety data sheet | See: data page | ||
| R/S statement (outdated) | R: ? S: ? | ||
| NFPA 704 | |||
| Flash point | N/A | ||
| Related compounds | |||
| Related halocarbons |
Tetrafluoromethane Hexafluoroethane | ||
| Related compounds |
Propane | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
| Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Octafluoropropane (C3F8) is a fluorocarbon non-flammable greenhouse gas that can be produced either by electrochemical fluorination or by the Fowler process using cobalt fluoride.[1]
Applications
In the electronics industry, octafluoropropane is mixed with oxygen and used as a plasma etching material for SiO2 layers in semiconductor applications, as oxides are selectively etched versus their metal substrates.[2]
In medicine, octafluoropropane may compose the gas cores of microbubble contrast agents used in contrast-enhanced ultrasound. Octafluoropropane microbubbles reflects sound waves well and are used to improve the ultrasound signal backscatter. It is also used in pars plana vitrectomy procedures where a retina hole or tear is repaired. The gas acts to provide a long-term tamponade, or plug, of a retinal hole/tear and allows re-attachment of the retina to occur over the following several days post-op.
Under the name R-218, octafluoropropane is used in other industries as a component of refrigeration mixtures.
It has featured in some plans for terraforming Mars.[3]
It is the active liquid in PICO-2L dark matter bubble detector (joined PICASSO and COUPP collaborations).
Liquid phase
- Liquid density (1.013 bar at boiling point) : 1601 kg/m3
- Liquid/gas equivalent (1.013 bar and 15 °C (59 °F)) : 196 vol/vol
- Latent heat of vaporization (1.013 bar at boiling point) : 104.25 kJ/kg [4]
Gaseous phase
- Gas density (1.013 bar at boiling point) : 10.3 kg/m3
- Gas density (1.013 bar and 15 °C (59 °F)) : 8.17 kg/m3
- Compressibility Factor (Z) (1.013 bar and 15 °C (59 °F)) : 0.975
- Specific gravity (air = 1) (1.013 bar and 21 °C (70 °F)) : 6.683
- Specific volume (1.013 bar and 21 °C (70 °F)) : 0.125 m3/kg
- Viscosity (1.013 bar and 0 °C (32 °F)) : 0.000125 Poise
- Thermal conductivity (1.013 bar and 0 °C (32 °F)) : 12.728 mW/(m·K)
- Thermal Conductivity, Gas @ 101.325 kPa and 25 °C: 13.8 mW/(m·K)
- Vapour Pressure @ 21.1 °C: 792 kPa [4]
Major hazards
References
- ↑ Fowler RD, Buford III WB, Hamilton Jr JM, Sweet RG, Weber CE, Kasper JS, Litant I (1947). "Synthesis of Fluorocarbons". Ind. Eng. Chem. 39 (3): 292–298. doi:10.1021/ie50447a612.
- ↑ Coburn, J. W. (1982). "Plasma-assisted etching". Plasma Chemistry and Plasma Processing. 2 (1): 1–41. doi:10.1007/BF00566856.
- ↑ D. Rogers (17–21 October 2005). Studies in the Future of Experimental Terraforming (PDF). 56th International Astronautical Congress of the International Astronautical Federation. Fukuoka, Japan: International Academy of Astronautics, and the International Institute of Space Law.
- 1 2 "Encyclopédie des gaz". air liquide.