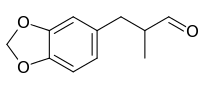
Helional
 | |
| Names | |
|---|---|
| IUPAC name
3-(1,3-Benzodioxol-5-yl)-2-methylpropanal | |
| Other names
2-Methyl-3-(3,4-methylenedioxyphenyl)propanal; 2-piperonylpropanal Helional Ocean propanal | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.528 |
| PubChem CID |
|
| |
| |
| Properties | |
| C11H12O3 | |
| Molar mass | 192.21 g·mol−1 |
| Appearance | clear colorless liquid |
| Odor | floral, herbaceous |
| Density | 1.162 g/ml |
| Boiling point | 282°C |
| Hazards | |
| Safety data sheet | [1] |
| GHS pictograms |  |
| GHS signal word | Warning |
| H315, H319, H335 | |
| P261, P305+351+338 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Helional (from heliotropin, from which is it commonly derived) is a chemical compound used as a perfume in soap and laundry detergent. Chemically it is an aldehyde with a dihydrocinnamaldehyde motif; a structural element which is present in a number of other important commercial fragrances and odorants.[2]
Synthesis
Several synthetic routes exist but the most common is a crossed-aldol condensation between piperonal (heliotropin) and propanal followed by selective hydrogenation of the intermediate alkene. This produces a racemic product.
See also
References
- ↑ Sigma-Aldrich Co., 2-methyl-3-(3,4-methylenedioxyphenyl)propanal. Retrieved on 2016-03-02.
- ↑ Wetzel, Christian H.; Oles, Markus; Wellerdieck, Christiane; Kuczkowiak, Michael; Gisselmann, Gunter; Hatt, Hanns (1999). "Specificity and sensitivity of a human olfactory receptor functionally expressed in human embryonic kidney 293 cells and Xenopus laevis oocytes". Journal of Neuroscience. 19: 7426–7433. doi:10.1093/chemse/bji002.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.