Nucleus accumbens
| Nucleus accumbens | |
|---|---|
 Medial surface, person facing to the left. Nucleus accumbens is very roughly in Brodmann area 34 | |
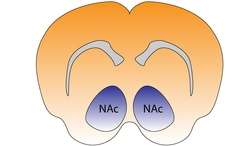 Nucleus accumbens of the mouse brain | |
| Details | |
| Part of |
Mesolimbic pathway Basal ganglia (Ventral striatum) |
| Components |
Nucleus accumbens shell Nucleus accumbens core |
| Identifiers | |
| Latin | nucleus accumbens septi |
| Acronym(s) | NAc or NAcc |
| MeSH | A08.186.211.730.885.105.683 |
| NeuroNames | hier-259 |
| NeuroLex ID | Nucleus accumbens |
| Dorlands /Elsevier | n_11/12580142 |
| TA | A14.1.09.440 |
| FMA | 61889 |
The nucleus accumbens (NAc or NAcc), also known as the accumbens nucleus or as the nucleus accumbens septi (Latin for nucleus adjacent to the septum) is a region in the basal forebrain rostral to the preoptic area of the hypothalamus.[1] The nucleus accumbens and the olfactory tubercle collectively form the ventral striatum, which is part of the striatum the main component of the basal ganglia.[2] The dopaminergic neurons of the mesolimbic pathway project onto the GABAergic medium spiny neurons of the nucleus accumbens and olfactory tubercle.[3][4] Each cerebral hemisphere has its own nucleus accumbens, which can be divided into two structures: the nucleus accumbens core and the nucleus accumbens shell. These substructures have different morphology and functions.
Different NAcc subregions (core vs shell) and neuron subpopulations within each region (D1-type vs D2-type medium spiny neurons) are responsible for different cognitive functions.[5][6] As a whole, the nucleus accumbens has a significant role in the cognitive processing of aversion, motivation, reward (i.e., incentive salience, pleasure, and positive reinforcement), and reinforcement learning;[4][7][8] hence, it has a significant role in addiction.[4][8] It plays a lesser role in processing fear (a form of aversion), impulsivity, and the placebo effect.[9][10][11] It is involved in the encoding of new motor programs as well.[4]
Structure
The nucleus accumbens is an aggregate of neurons which is described as having an outer shell and an inner core.[4]
Input
Major glutamatergic inputs to the nucleus accumbens include the prefrontal cortex (particularly the prelimbic cortex and infralimbic cortex), basolateral amygdala, ventral hippocampus, thalamic nuclei (specifically the midline thalamic nuclei and intralaminar nuclei of the thalamus), and glutamatergic projections from the ventral tegmental area.[12] The nucleus accumbens receives dopaminergic inputs from the ventral tegmental area (VTA), which connect via the mesolimbic pathway. The nucleus accumbens is often described as one part of a cortico-basal ganglia-thalamo-cortical loop.[13]
Dopaminergic inputs from the VTA modulate the activity of GABAergic neurons within the nucleus accumbens. These neurons are activated directly or indirectly by euphoriant drugs (e.g., amphetamine, opiates, etc.) and by participating in rewarding experiences (e.g., sex, music, exercise, etc.).[14][15]
Another major source of input comes from the CA1 and ventral subiculum of the hippocampus to the dorsomedial area of the nucleus accumbens. Slight depolarizations of cells in the nucleus accumbens correlates with positivity of the neurons of the hippocampus, making them more excitable. The correlated cells of these excited states of the medium spiny neurons in the nucleus accumbens are shared equally between the subiculum and CA1. The subiculum neurons are found to hyperpolarize (increase negativity) while the CA1 neurons "ripple" (fire > 50 Hz) in order to accomplish this priming.[16]
The nucleus accumbens is one of the few regions that receive histaminergic projections from the tuberomammillary nucleus (the sole source of histamine neurons in the brain).[17]
Output
The output neurons of the nucleus accumbens send axonal projections to the basal ganglia and the ventral analog of the globus pallidus, known as the ventral pallidum (VP). The VP, in turn, projects to the medial dorsal nucleus of the dorsal thalamus, which projects to the prefrontal cortex as well as the striatum. Other efferents from the nucleus accumbens include connections with the tail of the ventral tegmental area,[18] substantia nigra, and the reticular formation of the pons.[1]
Shell
The nucleus accumbens shell (NAcc shell) is a substructure of the nucleus accumbens. The shell and core together form the entire nucleus accumbens.
Location: The shell is the outer region of the nucleus accumbens, and – unlike the core – is considered to be part of the extended amygdala, located at its rostral pole.
Cell types: Neurons in the nucleus accumbens are mostly medium spiny neurons (MSNs) containing mainly D1-type (i.e., DRD1 and DRD5) or D2-type (i.e., DRD2, DRD3, and DRD4) dopamine receptors. A subpopulation of MSNs contain both D1-type and D2-type receptors, with approximately 40% of striatal MSNs expressing both DRD1 and DRD2 mRNA.[13][19][20] These mixed-type NAcc MSNs with both D1-type and D2-type receptors are mostly confined to the NAcc shell.[13] The neurons in the shell, as compared to the core, have a lower density of dendritic spines, less terminal segments, and less branch segments than those in the core. The shell neurons project to the subcommissural part of the ventral pallidum as well as the ventral tegmental area and to extensive areas in the hypothalamus and extended amygdala.[21][22][23]
Function: The shell of the nucleus accumbens is involved in the cognitive processing of reward, including subjective "liking" reactions to certain pleasurable stimuli, motivational salience, and positive reinforcement.[4][5][24][25] That NAcc shell has also been shown to mediate specific Pavlovian-instrumental transfer, a phenomenon in which a classically conditioned stimulus modifies operant behavior.[26][27][28] A "hedonic hotspot" or pleasure center which is responsible for the pleasurable or "liking" component of some intrinsic rewards is also located in a small compartment within the medial NAcc shell.[24][29][30] The D1-type medium spiny neurons in the Nacc shell mediate reward-related cognitive processes,[5][31][32] whereas the D2-type medium spiny neurons in the NAcc shell mediate aversion-related cognition.[6] Addictive drugs have a larger effect on dopamine release in the shell than in the core.[4]
Core
The nucleus accumbens core (NAcc core) is the inner substructure of the nucleus accumbens.
Location: The nucleus accumbens core is part of the ventral striatum, located within the basal ganglia.
Cell types: The core of the NAcc is made up mainly of medium spiny neurons containing mainly D1-type or D2-type dopamine receptors. The neurons in the core, as compared to the neurons in the shell, have an increased density of dendritic spines, branch segments, and terminal segments. From the core, the neurons project to other sub-cortical areas such as the globus pallidus and the substantia nigra. GABA is one of the main neurotransmitters in the NAcc, and GABA receptors are also abundant.[21][23]
Function: The nucleus accumbens core is involved in the cognitive processing of motor function related to reward and reinforcement.[4] Specifically, the core encodes new motor programs which facilitate the acquisition of a given reward in the future.[4] The NAcc core has been shown to mediate general Pavlovian-instrumental transfer, a phenomenon in which a classically conditioned stimulus modifies operant behavior.[26][27][28]
Cell types
Approximately 95% of neurons in the NAcc are GABAergic medium spiny neurons (MSNs) which primarily express either D1-type or D2-type receptors;[14] about 1–2% of the remaining neuronal types are large aspiny cholinergic interneurons and another 1–2% are GABAergic interneurons.[14] Compared to the GABAergic MSNs in the shell, those in the core have an increased density of dendritic spines, branch segments, and terminal segments. From the core, the neurons project to other sub-cortical areas such as the globus pallidus and the substantia nigra. GABA is one of the main neurotransmitters in the NAcc, and GABA receptors are also abundant.[21][23] These neurons are also the main projection or output neurons of the nucleus accumbens.
Neurotransmitters
Dopamine: Dopamine is released into the nucleus accumbens following exposure to rewarding stimuli, including recreational drugs like substituted amphetamines, cocaine, and morphine.[33][34]
Phenethylamine and tyramine: Phenethylamine and tyramine are trace amine compounds which are synthesized in several types of CNS neurons, including all dopamine neurons.[35] Specifically, these neurotransmitters act within the dopaminergic inputs to the NAcc. These substances regulate the presynaptic release of dopamine through their interactions with VMAT2 and TAAR1, analogous to amphetamine.
Glucocorticoids and dopamine: Glucocorticoid receptors are the only corticosteroid receptors in the nucleus accumbens shell. L-DOPA, steroids, and specifically glucocorticoids are currently known to be the only known endogenous compounds that can induce psychotic problems, so understanding the hormonal control over dopaminergic projections with regards to glucocorticoid receptors could lead to new treatments for psychotic symptoms. A recent study demonstrated that suppression of the glucocorticoid receptors led to a decrease in the release of dopamine, which may lead to future research involving anti-glucocorticoid drugs to potentially relieve psychotic symptoms.[36]
GABA: A recent study on rats that used GABA agonists and antagonists indicated that GABAA receptors in the NAc shell have inhibitory control on turning behavior influenced by dopamine, and GABAB receptors have inhibitory control over turning behavior mediated by acetylcholine.[21][37]
Glutamate: Studies have shown that local blockade of glutamatergic NMDA receptors in the NAcc core impaired spatial learning.[38] Another study demonstrated that both NMDA and AMPA (both glutamate receptors) play important roles in regulating instrumental learning.[39]
Serotonin (5-HT): Overall, 5-HT synapses are more abundant and have a greater number of synaptic contacts in the NAc shell than in the core. They are also larger and thicker, and contain more large dense core vesicles than their counterparts in the core.
Function
Reward and reinforcement
The nucleus accumbens, being one part of the reward system, plays an important role in processing rewarding stimuli, reinforcing stimuli (e.g., food and water), and those which are both rewarding and reinforcing (addictive drugs, sex, and exercise).[4][40] The nucleus accumbens is selectively activated during the perception of pleasant, emotionally arousing pictures and during mental imagery of pleasant, emotional scenes.[41][42] A 2005 study found that it is involved in the regulation of emotions induced by music,[43] perhaps consequent to its role in mediating dopamine release. The nucleus accumbens plays a role in rhythmic timing and is considered to be of central importance to the limbic-motor interface (Mogensen).
In the 1950s, James Olds and Peter Milner implanted electrodes into the septal area of the rat and found that the rat chose to press a lever which stimulated it. It continued to prefer this even over stopping to eat or drink. This suggests that the area is the "pleasure center" of the brain and is involved in reinforcement learning.[44] In rats, stimulation of the ventral tegmental area causes the release of dopamine in the nucleus accumbens much in the same way as addictive drugs and natural reinforcers, such as water or food, initiate the release of dopamine in the nucleus accumbens.[45] The same results have been seen in human subjects in functional imaging studies. For example, increased dopamine concentration is seen in the extracellular fluid of the nucleus accumbens when subjects believed they were being given money, and increased activation (i.e., increased fMRI BOLD signal-change) was observed among heterosexual males viewing pictures of attractive women.[46]
Aversion
Activation of D1-type MSNs in the nucleus accumbens is involved in reward, whereas the activation of D2-type MSNs in the nucleus accumbens promotes aversion.[6]
Maternal behavior
An fMRI study conducted in 2005 found that when mother rats were in the presence of their pups the regions of the brain involved in reinforcement, including the nucleus accumbens, were highly active.[47] Levels of dopamine increase in the nucleus accumbens during maternal behavior, while lesions in this area upset maternal behavior.[48] When women are presented pictures of unrelated infants, fMRIs show increased brain activity in the nucleus accumbens and adjacent caudate nucleus, proportionate to the degree to which the women find these infants "cute".[49]
Clinical significance
Addiction
Current models of addiction from chronic drug use involve alterations in gene expression in the mesocorticolimbic projection.[14][50][51] The most important transcription factors that produce these alterations are ΔFosB, cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), and nuclear factor kappa B (NFκB).[14] ΔFosB is the most significant gene transcription factor in addiction since its viral or genetic overexpression in the nucleus accumbens is necessary and sufficient for many of the neural adaptations and behavioral effects (e.g., expression-dependent increases in self-administration and reward sensitization) seen in drug addiction.[14][31][52] ΔFosB overexpression has been implicated in addictions to alcohol (ethanol), cannabinoids, cocaine, methylphenidate, nicotine, opioids, phencyclidine, propofol, and substituted amphetamines, among others.[14][50][52][53][54] Increases in nucleus accumbens ΔJunD expression can reduce or, with a large increase, even block most of the neural alterations seen in chronic drug abuse (i.e., the alterations mediated by ΔFosB).[14]
ΔFosB also plays an important role in regulating behavioral responses to natural rewards, such as palatable food, sex, and exercise.[14][15] Natural rewards, like drugs of abuse, induce ΔFosB in the nucleus accumbens, and chronic acquisition of these rewards can result in a similar pathological addictive state through ΔFosB overexpression.[14][15][40] Consequently, ΔFosB is the key transcription factor involved in addictions to natural rewards as well;[14][15][40] in particular, ΔFosB in the nucleus accumbens is critical for the reinforcing effects of sexual reward.[15] Research on the interaction between natural and drug rewards suggests that psychostimulants and sexual behavior act on similar biomolecular mechanisms to induce ΔFosB in the nucleus accumbens and possess cross-sensitization effects that are mediated through ΔFosB.[40][55]
Similar to drug rewards, non-drug rewards also increase the level of extracellular dopamine in the NAcc shell. Drug-induced dopamine release in the NAcc shell and NAcc core is usually not prone to habituation (i.e., the development of drug tolerance: a decrease in dopamine release from future drug exposure as a result of repeated drug exposure); on the contrary, repeated exposure to drugs that induce dopamine release in the NAcc shell and core typically results in sensitization (i.e., the amount of dopamine that is released in the NAcc from future drug exposure increases as a result of repeated drug exposure). Sensitization of dopamine release in the NAcc shell following repeated drug exposure serves to strengthen stimulus-drug associations (i.e., classical conditioning that occurs when drug use is repeatedly paired with environmental stimuli) and these associations become less prone to extinction (i.e., "unlearning" these classically conditioned associations between drug use and environmental stimuli becomes more difficult). After repeated pairing, these classically conditioned environmental stimuli (e.g., contexts and objects that are frequently paired with drug use) often become drug cues which function as secondary reinforcers of drug use (i.e., once these associations are established, exposure to a paired environmental stimulus triggers a craving or desire to use the drug which they've become associated with).[21][34]
In contrast to drugs, the release of dopamine in the NAcc shell by many types of rewarding non-drug stimuli typically undergoes habituation following repeated exposure (i.e., the amount of dopamine that is released from future exposure to a rewarding non-drug stimulus normally decreases as a result of repeated exposure to that stimulus).[21][34]
| Form of neuroplasticity or behavioral plasticity |
Type of reinforcer | Sources | |||||
|---|---|---|---|---|---|---|---|
| Opiates | Psychostimulants | High fat or sugar food | Sexual intercourse | Physical exercise (aerobic) |
Environmental enrichment | ||
| ΔFosB expression in nucleus accumbens D1-type MSNs |
↑ | ↑ | ↑ | ↑ | ↑ | ↑ | [40] |
| Behavioral plasticity | |||||||
| Escalation of intake | Yes | Yes | Yes | [40] | |||
| Psychostimulant cross-sensitization |
Yes | Not applicable | Yes | Yes | Attenuated | Attenuated | [40] |
| Psychostimulant self-administration |
↑ | ↑ | ↓ | ↓ | ↓ | [40] | |
| Psychostimulant conditioned place preference |
↑ | ↑ | ↓ | ↑ | ↓ | ↑ | [40] |
| Reinstatement of drug-seeking behavior | ↑ | ↑ | ↓ | ↓ | [40] | ||
| Neurochemical plasticity | |||||||
| CREB phosphorylation in the nucleus accumbens |
↓ | ↓ | ↓ | ↓ | ↓ | [40] | |
| Sensitized dopamine response in the nucleus accumbens |
No | Yes | No | Yes | [40] | ||
| Altered striatal dopamine signaling | ↓DRD2, ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD2 | ↑DRD2 | [40] | |
| Altered striatal opioid signaling | No change or ↑μ-opioid receptors | ↑μ-opioid receptors ↑κ-opioid receptors | ↑μ-opioid receptors | ↑μ-opioid receptors | No change | No change | [40] |
| Changes in striatal opioid peptides | ↑dynorphin No change: enkephalin | ↑dynorphin | ↓enkephalin | ↑dynorphin | ↑dynorphin | [40] | |
| Mesocorticolimbic synaptic plasticity | |||||||
| Number of dendrites in the nucleus accumbens | ↓ | ↑ | ↑ | [40] | |||
| Dendritic spine density in the nucleus accumbens |
↓ | ↑ | ↑ | [40] | |||
Depression
In April 2007, two research teams reported on having inserted electrodes into the nucleus accumbens in order to use deep brain stimulation to treat severe depression.[56] In 2010, experiments reported that deep brain stimulation of the nucleus accumbens was successful in decreasing depression symptoms in 50% of patients who did not respond to other treatments such as electroconvulsive therapy.[57] Nucleus accumbens has also been used as a target to treat small groups of patients with therapy-refractory obsessive-compulsive disorder.[58]
Ablation
To treat addiction and in an attempt to treat mental illness radiofrequency ablation of the nucleus accumbens has been performed. The results are inconclusive and controversial.[59][60]
Placebo effect
Activation of the NAcc has been shown to occur in the anticipation of effectiveness of a drug when a user is given a placebo, indicating a contributing role of the nucleus accumbens in the placebo effect.[10][61]
Additional images
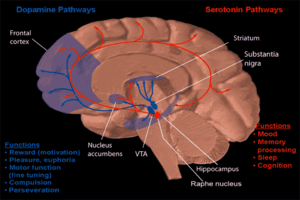 Dopamine and serotonin
Dopamine and serotonin- MRI coronal slice showing nucleus accumbens outlined in red
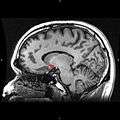 Sagittal MRI slice with highlighting (red) indicating the nucleus accumbens.
Sagittal MRI slice with highlighting (red) indicating the nucleus accumbens.
See also
References
- 1 2 Carlson, Neil R. Physiology of Behavior. 11th ed. Boston: Pearson, 2013. Print.
- ↑ Nucleus Accumbens
- ↑ Ikemoto S (2010). "Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory". Neurosci Biobehav Rev. 35 (2): 129–50. PMC 2894302
 . PMID 20149820. doi:10.1016/j.neubiorev.2010.02.001.
. PMID 20149820. doi:10.1016/j.neubiorev.2010.02.001. Recent studies on intracranial self-administration of neurochemicals (drugs) found that rats learn to self-administer various drugs into the mesolimbic dopamine structures–the posterior ventral tegmental area, medial shell nucleus accumbens and medial olfactory tubercle. ... In the 1970s it was recognized that the olfactory tubercle contains a striatal component, which is filled with GABAergic medium spiny neurons receiving glutamatergic inputs form cortical regions and dopaminergic inputs from the VTA and projecting to the ventral pallidum just like the nucleus accumbens
Figure 3: The ventral striatum and self-administration of amphetamine - 1 2 3 4 5 6 7 8 9 10 Malenka RC, Nestler EJ, Hyman SE (2009). Sydor A, Brown RY, eds. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 147–148, 367, 376. ISBN 978-0-07-148127-4.
VTA DA neurons play a critical role in motivation, reward-related behavior (Chapter 15), attention, and multiple forms of memory. This organization of the DA system, wide projection from a limited number of cell bodies, permits coordinated responses to potent new rewards. Thus, acting in diverse terminal fields, dopamine confers motivational salience (“wanting”) on the reward itself or associated cues (nucleus accumbens shell region), updates the value placed on different goals in light of this new experience (orbital prefrontal cortex), helps consolidate multiple forms of memory (amygdala and hippocampus), and encodes new motor programs that will facilitate obtaining this reward in the future (nucleus accumbens core region and dorsal striatum). In this example, dopamine modulates the processing of sensorimotor information in diverse neural circuits to maximize the ability of the organism to obtain future rewards. ...
The brain reward circuitry that is targeted by addictive drugs normally mediates the pleasure and strengthening of behaviors associated with natural reinforcers, such as food, water, and sexual contact. Dopamine neurons in the VTA are activated by food and water, and dopamine release in the NAc is stimulated by the presence of natural reinforcers, such as food, water, or a sexual partner. ...
The NAc and VTA are central components of the circuitry underlying reward and memory of reward. As previously mentioned, the activity of dopaminergic neurons in the VTA appears to be linked to reward prediction. The NAc is involved in learning associated with reinforcement and the modulation of motoric responses to stimuli that satisfy internal homeostatic needs. The shell of the NAc appears to be particularly important to initial drug actions within reward circuitry; addictive drugs appear to have a greater effect on dopamine release in the shell than in the core of the NAc. - 1 2 3 Saddoris MP, Cacciapaglia F, Wightman RM, Carelli RM (August 2015). "Differential Dopamine Release Dynamics in the Nucleus Accumbens Core and Shell Reveal Complementary Signals for Error Prediction and Incentive Motivation". J. Neurosci. 35 (33): 11572–82. PMC 4540796
 . PMID 26290234. doi:10.1523/JNEUROSCI.2344-15.2015.
. PMID 26290234. doi:10.1523/JNEUROSCI.2344-15.2015. Here, we have found that real-time dopamine release within the nucleus accumbens (a primary target of midbrain dopamine neurons) strikingly varies between core and shell subregions. In the core, dopamine dynamics are consistent with learning-based theories (such as reward prediction error) whereas in the shell, dopamine is consistent with motivation-based theories (e.g., incentive salience).
- 1 2 3 Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Peña CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, Deisseroth K, Nestler EJ (February 2016). "In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward". Proc. Natl. Acad. Sci. U.S.A. 113: 2726–31. PMID 26831103. doi:10.1073/pnas.1521238113.
Previous work has demonstrated that optogenetically stimulating D1 MSNs promotes reward, whereas stimulating D2 MSNs produces aversion.
- ↑ Wenzel JM, Rauscher NA, Cheer JF, Oleson EB (2015). "A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature". ACS Chem Neurosci. 6 (1): 16–26. PMID 25491156. doi:10.1021/cn500255p.
- 1 2 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 10: Neural and Neuroendocrine Control of the Internal Milieu". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 266. ISBN 978-0-07-148127-4.
Dopamine acts in the nucleus accumbens to attach motivational significance to stimuli associated with reward.
- ↑ Schwienbacher, Isabel; Fendt, Markus; Richardson, Rick; Schnitzler, Hans-Ulrich (2004). "Temporary inactivation of the nucleus accumbens disrupts acquisition and expression of fear-potentiated startle in rats". Brain Research. 1027 (1–2): 87–93. PMID 15494160. doi:10.1016/j.brainres.2004.08.037.
- 1 2 Zubieta, Jon-Kar; Stohler, Christian S. (2009). "Neurobiological Mechanisms of Placebo Responses". Annals of the New York Academy of Sciences. 1156: 198–210. Bibcode:2009NYASA1156..198Z. PMC 3073412
 . PMID 19338509. doi:10.1111/j.1749-6632.2009.04424.x.
. PMID 19338509. doi:10.1111/j.1749-6632.2009.04424.x. - ↑ Basar, Koray; Sesia, Thibaut; Groenewegen, Henk; Steinbusch, Harry W.M.; Visser-Vandewalle, Veerle; Temel, Yasin (2010). "Nucleus accumbens and impulsivity". Progress in Neurobiology. 92 (4): 533–57. PMID 20831892. doi:10.1016/j.pneurobio.2010.08.007.
- ↑ Gipson CD, Kupchik YM, Kalivas PW (January 2014). "Rapid, transient synaptic plasticity in addiction". Neuropharmacology. 76 Pt B: 276–286. PMC 3762905
 . PMID 23639436. doi:10.1016/j.neuropharm.2013.04.032.
. PMID 23639436. doi:10.1016/j.neuropharm.2013.04.032. Within a simplified PFC-NAc-VTA circuit, the NAc serves as a “gateway” through which information regarding the direction of behavioral output is processed from limbic cortex to motor subcircuits. It is thought that the transition to compulsive drug seeking arises from an impaired ability of this subcircuit to effectively process information about negative environmental contingencies, leading to an inability to inhibit prepotent drug-associated responses; thereby the addict is rendered prone to relapse.
Figure 1: Glutamatergic afferents to the nucleus accumbens involved in addictive behavior - 1 2 3 Yager LM, Garcia AF, Wunsch AM, Ferguson SM (August 2015). "The ins and outs of the striatum: Role in drug addiction". Neuroscience. 301: 529–541. PMC 4523218
 . PMID 26116518. doi:10.1016/j.neuroscience.2015.06.033.
. PMID 26116518. doi:10.1016/j.neuroscience.2015.06.033. [The striatum] receives dopaminergic inputs from the ventral tegmental area (VTA) and the substantia nigra (SNr) and glutamatergic inputs from several areas, including the cortex, hippocampus, amygdala, and thalamus (Swanson, 1982; Phillipson and Griffiths, 1985; Finch, 1996; Groenewegen et al., 1999; Britt et al., 2012). These glutamatergic inputs make contact on the heads of dendritic spines of the striatal GABAergic medium spiny projection neurons (MSNs) whereas dopaminergic inputs synapse onto the spine neck, allowing for an important and complex interaction between these two inputs in modulation of MSN activity ... It should also be noted that there is a small population of neurons in the NAc that coexpress both D1 and D2 receptors, though this is largely restricted to the NAc shell (Bertran- Gonzalez et al., 2008). ... Neurons in the NAc core and NAc shell subdivisions also differ functionally. The NAc core is involved in the processing of conditioned stimuli whereas the NAc shell is more important in the processing of unconditioned stimuli; Classically, these two striatal MSN populations are thought to have opposing effects on basal ganglia output. Activation of the dMSNs causes a net excitation of the thalamus resulting in a positive cortical feedback loop; thereby acting as a ‘go’ signal to initiate behavior. Activation of the iMSNs, however, causes a net inhibition of thalamic activity resulting in a negative cortical feedback loop and therefore serves as a ‘brake’ to inhibit behavior ... there is also mounting evidence that iMSNs play a role in motivation and addiction (Lobo and Nestler, 2011; Grueter et al., 2013). ... Together these data suggest that iMSNs normally act to restrain drug-taking behavior and recruitment of these neurons may in fact be protective against the development of compulsive drug use.
- 1 2 3 4 5 6 7 8 9 10 11 Robison, Alfred J.; Nestler, Eric J. (2011). "Transcriptional and epigenetic mechanisms of addiction". Nature Reviews Neuroscience. 12 (11): 623–37. PMC 3272277
 . PMID 21989194. doi:10.1038/nrn3111.
. PMID 21989194. doi:10.1038/nrn3111. ΔFosB has been linked directly to several addiction-related behaviors ... Importantly, genetic or viral overexpression of ΔJunD, a dominant negative mutant of JunD which antagonizes ΔFosB- and other AP-1-mediated transcriptional activity, in the NAc or OFC blocks these key effects of drug exposure14,22–24. This indicates that ΔFosB is both necessary and sufficient for many of the changes wrought in the brain by chronic drug exposure. ΔFosB is also induced in D1-type NAc MSNs by chronic consumption of several natural rewards, including sucrose, high fat food, sex, wheel running, where it promotes that consumption14,26–30. This implicates ΔFosB in the regulation of natural rewards under normal conditions and perhaps during pathological addictive-like states. ... 95% of NAc neurons are GABAergic MSNs (medium spiny neurons), which can be further differentiated into those MSNs that express the D1 dopamine receptor (D1-type MSNs) and express dynorphin and substance P and those that express the D2 dopamine receptor (D2-type MSNs) and express enkephalin132. Drug induction of ΔFosB133,134, and the effects of ΔFosB and G9a on cell morphology and behavior, differ between D1-type and D2-type MSNs135, and neuronal activity of these two cell types causes opposing effects on the rewarding properties of cocaine131. ... About 1–2% of NAc neurons are aspiny large cholinergic interneurons, which have been shown to play an important role in cocaine reward130, and a similar number are GABAergic interneurons, the function of which are less well understood.
- 1 2 3 4 5 Blum, Kenneth; Werner, Tonia; Carnes, Stefanie; Carnes, Patrick; Bowirrat, Abdalla; Giordano, John; Marlene-Oscar-Berman; Gold, Mark (2012). "Sex, Drugs, and Rock 'N' Roll: Hypothesizing Common Mesolimbic Activation as a Function of Reward Gene Polymorphisms". Journal of Psychoactive Drugs. 44 (1): 38–55. PMC 4040958
 . PMID 22641964. doi:10.1080/02791072.2012.662112.
. PMID 22641964. doi:10.1080/02791072.2012.662112. It has been found that deltaFosB gene in the NAc is critical for reinforcing effects of sexual reward. Pitchers and colleagues (2010) reported that sexual experience was shown to cause DeltaFosB accumulation in several limbic brain regions including the NAc, medial pre-frontal cortex, VTA, caudate, and putamen, but not the medial preoptic nucleus. Next, the induction of c-Fos, a downstream (repressed) target of DeltaFosB, was measured in sexually experienced and naive animals. The number of mating-induced c-Fos-IR cells was significantly decreased in sexually experienced animals compared to sexually naive controls. Finally, DeltaFosB levels and its activity in the NAc were manipulated using viral-mediated gene transfer to study its potential role in mediating sexual experience and experience-induced facilitation of sexual performance. Animals with DeltaFosB overexpression displayed enhanced facilitation of sexual performance with sexual experience relative to controls. In contrast, the expression of DeltaJunD, a dominant-negative binding partner of DeltaFosB, attenuated sexual experience-induced facilitation of sexual performance, and stunted long-term maintenance of facilitation compared to DeltaFosB overexpressing group. Together, these findings support a critical role for DeltaFosB expression in the NAc in the reinforcing effects of sexual behavior and sexual experience-induced facilitation of sexual performance. ... both drug addiction and sexual addiction represent pathological forms of neuroplasticity along with the emergence of aberrant behaviors involving a cascade of neurochemical changes mainly in the brain's rewarding circuitry.
- ↑ Goto, Y; O'Donnell, P (2001). "Synchronous activity in the hippocampus and nucleus accumbens in vivo". The Journal of Neuroscience. 21 (4): RC131. PMID 11160416.
- ↑ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 175–176. ISBN 978-0-07-148127-4.
Within the brain, histamine is synthesized exclusively by neurons with their cell bodies in the tuberomammillary nucleus (TMN) that lies within the posterior hypothalamus. There are approximately 64000 histaminergic neurons per side in humans. These cells project throughout the brain and spinal cord. Areas that receive especially dense projections include the cerebral cortex, hippocampus, neostriatum, nucleus accumbens, amygdala, and hypothalamus. ... While the best characterized function of the histamine system in the brain is regulation of sleep and arousal, histamine is also involved in learning and memory ... It also appears that histamine is involved in the regulation of feeding and energy balance.
- ↑ Barrot, M.; Sesack, S. R.; Georges, F.; Pistis, M.; Hong, S.; Jhou, T. C. (2012). "Braking Dopamine Systems: A New GABA Master Structure for Mesolimbic and Nigrostriatal Functions". Journal of Neuroscience. 32 (41): 14094–101. PMC 3513755
 . PMID 23055478. doi:10.1523/JNEUROSCI.3370-12.2012.
. PMID 23055478. doi:10.1523/JNEUROSCI.3370-12.2012. - ↑ Ferré S, Lluís C, Justinova Z, Quiroz C, Orru M, Navarro G, Canela EI, Franco R, Goldberg SR (June 2010). "Adenosine-cannabinoid receptor interactions. Implications for striatal function". Br. J. Pharmacol. 160 (3): 443–453. PMC 2931547
 . PMID 20590556. doi:10.1111/j.1476-5381.2010.00723.x.
. PMID 20590556. doi:10.1111/j.1476-5381.2010.00723.x. Two classes of MSNs, which are homogeneously distributed in the striatum, can be differentiated by their output connectivity and their expression of dopamine and adenosine receptors and neuropeptides. In the dorsal striatum (mostly represented by the nucleus caudate-putamen), enkephalinergic MSNs connect the striatum with the globus pallidus (lateral globus pallidus) and express the peptide enkephalin and a high density of dopamine D2 and adenosine A2A receptors (they also express adenosine A1 receptors), while dynorphinergic MSNs connect the striatum with the substantia nigra (pars compacta and reticulata) and the entopeduncular nucleus (medial globus pallidus) and express the peptides dynorphin and substance P and dopamine D1 and adenosine A1 but not A2A receptors ... These two different phenotypes of MSN are also present in the ventral striatum (mostly represented by the nucleus accumbens and the olfactory tubercle). However, although they are phenotypically equal to their dorsal counterparts, they have some differences in terms of connectivity. First, not only enkephalinergic but also dynorphinergic MSNs project to the ventral counterpart of the lateral globus pallidus, the ventral pallidum, which, in fact, has characteristics of both the lateral and medial globus pallidus in its afferent and efferent connectivity. In addition to the ventral pallidum, the medial globus pallidus and the substantia nigra-VTA, the ventral striatum sends projections to the extended amygdala, the lateral hypothalamus and the pedunculopontine tegmental nucleus. ... It is also important to mention that a small percentage of MSNs have a mixed phenotype and express both D1 and D2 receptors (Surmeier et al., 1996).
- ↑ Nishi A, Kuroiwa M, Shuto T (July 2011). "Mechanisms for the modulation of dopamine d(1) receptor signaling in striatal neurons". Front Neuroanat. 5: 43. PMC 3140648
 . PMID 21811441. doi:10.3389/fnana.2011.00043.
. PMID 21811441. doi:10.3389/fnana.2011.00043. Dopamine plays critical roles in the regulation of psychomotor functions in the brain (Bromberg-Martin et al., 2010; Cools, 2011; Gerfen and Surmeier, 2011). The dopamine receptors are a superfamily of heptahelical G protein-coupled receptors, and are grouped into two categories, D1-like (D1, D5) and D2-like (D2, D3, D4) receptors, based on functional properties to stimulate adenylyl cyclase (AC) via Gs/olf and to inhibit AC via Gi/o, respectively ... It has been demonstrated that D1 receptors form the hetero-oligomer with D2 receptors, and that the D1–D2 receptor hetero-oligomer preferentially couples to Gq/PLC signaling (Rashid et al., 2007a,b). The expression of dopamine D1 and D2 receptors are largely segregated in direct and indirect pathway neurons in the dorsal striatum, respectively (Gerfen et al., 1990; Hersch et al., 1995; Heiman et al., 2008). However, some proportion of medium spiny neurons are known to expresses both D1 and D2 receptors (Hersch et al., 1995). Gene expression analysis using single cell RT-PCR technique estimated that 40% of medium spiny neurons express both D1 and D2 receptor mRNA (Surmeier et al., 1996).
- 1 2 3 4 5 6 Shirayama, Yukihiko; Chaki, Shigeyuki (2006). "Neurochemistry of the Nucleus Accumbens and Its Relevance to Depression and Antidepressant Action in Rodents". Current Neuropharmocology. 4 (4): 277–91. PMC 2475798
 . PMID 18654637. doi:10.2174/157015906778520773.
. PMID 18654637. doi:10.2174/157015906778520773. - ↑ Meredith, G.E.; Agolia, R.; Arts, M.P.M.; Groenewegen, H.J.; Zahm, D.S. (1992). "Morphological differences between projection neurons of the core and shell in the nucleus accumbens of the rat". Neuroscience. 50 (1): 149–62. PMID 1383869. doi:10.1016/0306-4522(92)90389-j.
- 1 2 3 Meredith, Gloria E.; Pennartz, Cyriel M.A.; Groenewegen, Henk J. (1993). "The cellular framework for chemical signalling in the nucleus accumbens". Chemical Signalling in the Basal Ganglia. Progress in Brain Research. 99. pp. 3–24. ISBN 978-0-444-81562-0. PMID 7906426. doi:10.1016/s0079-6123(08)61335-7.
- 1 2 Berridge KC, Kringelbach ML (May 2015). "Pleasure systems in the brain". Neuron. 86 (3): 646–664. PMC 4425246
 . PMID 25950633. doi:10.1016/j.neuron.2015.02.018.
. PMID 25950633. doi:10.1016/j.neuron.2015.02.018. - ↑ Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, Apkarian AV (October 2013). "Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain". J. Neurosci. 33 (41): 16383–16393. PMC 3792469
 . PMID 24107968. doi:10.1523/JNEUROSCI.1731-13.2013.
. PMID 24107968. doi:10.1523/JNEUROSCI.1731-13.2013. Recent evidence indicates that inactivation of D2 receptors, in the indirect striatopallidal pathway in rodents, is necessary for both acquisition and expression of aversive behavior, and direct pathway D1 receptor activation controls reward-based learning (Hikida et al., 2010; Hikida et al., 2013). It seems we can conclude that direct and indirect pathways of the NAc, via D1 and D2 receptors, subserve distinct anticipation and valuation roles in the shell and core of NAc, which is consistent with observations regarding spatial segregation and diversity of responses of midbrain dopaminergic neurons for rewarding and aversive conditions, some encoding motivational value, others motivational salience, each connected with distinct brain networks and having distinct roles in motivational control (Bromberg-Martin et al., 2010; Cohen et al., 2012; Lammel et al., 2013). ... Thus, the previous results, coupled with the current observations, imply that the NAc pshell response reflects a prediction/anticipation or salience signal, and the NAc pcore response is a valuation response (reward predictive signal) that signals the negative reinforcement value of cessation of pain (i.e., anticipated analgesia).
- 1 2 Cartoni E, Puglisi-Allegra S, Baldassarre G (November 2013). "The three principles of action: a Pavlovian-instrumental transfer hypothesis". Frontiers in Behavioral Neuroscience. 7: 153. PMC 3832805
 . PMID 24312025. doi:10.3389/fnbeh.2013.00153.
. PMID 24312025. doi:10.3389/fnbeh.2013.00153. - 1 2 Salamone JD, Pardo M, Yohn SE, López-Cruz L, SanMiguel N, Correa M (2016). "Mesolimbic Dopamine and the Regulation of Motivated Behavior". Current Topics in Behavioral Neurosciences. 27: 231–257. PMID 26323245. doi:10.1007/7854_2015_383.
Considerable evidence indicates that accumbens DA is important for Pavlovian approach and Pavlovian-to-instrumental transfer [(PIT)] ... PIT is a behavioral process that reflects the impact of Pavlovian-conditioned stimuli (CS) on instrumental responding. For example, presentation of a Pavlovian CS paired with food can increase output of food-reinforced instrumental behaviors, such as lever pressing. Outcome-specific PIT occurs when the Pavlovian unconditioned stimulus (US) and the instrumental reinforcer are the same stimulus, whereas general PIT is said to occur when the Pavlovian US and the reinforcer are different. ... More recent evidence indicates that accumbens core and shell appear to mediate different aspects of PIT; shell lesions and inactivation reduced outcome-specific PIT, while core lesions and inactivation suppressed general PIT (Corbit and Balleine 2011). These core versus shell differences are likely due to the different anatomical inputs and pallidal outputs associated with these accumbens subregions (Root et al. 2015). These results led Corbit and Balleine (2011) to suggest that accumbens core mediates the general excitatory effects of reward-related cues. PIT provides a fundamental behavioral process by which conditioned stimuli can exert activating effects upon instrumental responding
- 1 2 Corbit LH, Balleine BW (2016). "Learning and Motivational Processes Contributing to Pavlovian-Instrumental Transfer and Their Neural Bases: Dopamine and Beyond". Current Topics in Behavioral Neurosciences. 27: 259–289. PMID 26695169. doi:10.1007/7854_2015_388.
Such effects suggest that specific motivational states gate the arousing effects of Pavlovian incentives processes on instrumental performance ... Behavioral findings are supported by evidence that distinct neural circuits centered on the NAc core and shell mediate the general and specific forms of transfer, respectively, and ongoing work is beginning to explain how Pavlovian and instrumental learning processes that occur independently and at separate times are integrated within neural circuits that govern behavioral control.
- ↑ Richard JM, Castro DC, Difeliceantonio AG, Robinson MJ, Berridge KC (November 2013). "Mapping brain circuits of reward and motivation: in the footsteps of Ann Kelley". Neurosci. Biobehav. Rev. 37 (9 Pt A): 1919–1931. PMC 3706488
 . PMID 23261404. doi:10.1016/j.neubiorev.2012.12.008.
. PMID 23261404. doi:10.1016/j.neubiorev.2012.12.008.
Figure 3: Neural circuits underlying motivated 'wanting' and hedonic 'liking'. - ↑ Berridge KC, Robinson TE, Aldridge JW (2009). "Dissecting components of reward: 'liking', 'wanting', and learning". Current Opinion in Pharmacology. 9 (1): 65–73. PMC 2756052
 . PMID 19162544. doi:10.1016/j.coph.2008.12.014.
. PMID 19162544. doi:10.1016/j.coph.2008.12.014. - 1 2 Nestler EJ (December 2013). "Cellular basis of memory for addiction". Dialogues Clin. Neurosci. 15 (4): 431–443. PMC 3898681
 . PMID 24459410.
. PMID 24459410. DESPITE THE IMPORTANCE OF NUMEROUS PSYCHOSOCIAL FACTORS, AT ITS CORE, DRUG ADDICTION INVOLVES A BIOLOGICAL PROCESS: the ability of repeated exposure to a drug of abuse to induce changes in a vulnerable brain that drive the compulsive seeking and taking of drugs, and loss of control over drug use, that define a state of addiction. ... A large body of literature has demonstrated that such ΔFosB induction in D1-type NAc neurons increases an animal's sensitivity to drug as well as natural rewards and promotes drug self-administration, presumably through a process of positive reinforcement ... For example, the shell and core subregions of NAc display differences in drug-induced synaptic plasticity, as do D1- versus D2-type medium spiny neurons within each subregion.60,63,64,67
- ↑ Dumitriu D, Laplant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, Morrison JH, Nestler EJ (2012). "Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens". J. Neurosci. 32 (20): 6957–66. PMC 3360066
 . PMID 22593064. doi:10.1523/JNEUROSCI.5718-11.2012.
. PMID 22593064. doi:10.1523/JNEUROSCI.5718-11.2012. The enduring spine density change in core but not shell fits well with the established idea that the shell is preferentially involved in the development of addiction, while the core mediates the long-term execution of learned addiction-related behaviors (Ito et al., 2004; Di Chiara, 2002; Meredith et al., 2008). Consistent with the idea of NAc core being the locus of long-lasting drug-induced neuroplasticity, several studies have shown that electrophysiological changes in core persist longer than their shell counterparts. ... Furthermore, data presented here support the idea that NAc shell is preferentially involved in immediate drug reward, while the core might play a more explicit role in longer-term aspects of addiction.
- ↑ Pontieri, F. E.; Tanda, G.; Di Chiara, G. (1995). "Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the 'shell' as compared with the 'core' of the rat nucleus accumbens". Proceedings of the National Academy of Sciences. 92 (26): 12304–8. Bibcode:1995PNAS...9212304P. JSTOR 2369093. PMC 40345
 . PMID 8618890. doi:10.1073/pnas.92.26.12304.
. PMID 8618890. doi:10.1073/pnas.92.26.12304. - 1 2 3 Di Chiara, Gaetano (2002). "Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction". Behavioural Brain Research. 137 (1–2): 75–114. PMID 12445717. doi:10.1016/s0166-4328(02)00286-3.
- ↑ Eiden, Lee E.; Weihe, Eberhard (2011). "VMAT2: A dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Annals of the New York Academy of Sciences. 1216: 86–98. Bibcode:2011NYASA1216...86E. PMC 4183197
 . PMID 21272013. doi:10.1111/j.1749-6632.2010.05906.x.
. PMID 21272013. doi:10.1111/j.1749-6632.2010.05906.x. VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR) ... [Trace aminergic] neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC).
- ↑ Barrot, Michel; Marinelli, Michela; Abrous, Djoher Nora; Rouge-Pont, Francoise; Le Moal, Michel; Piazza, Pier Vincenzo (2000). "The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent". European Journal of Neuroscience. 12 (3): 973–9. PMID 10762327. doi:10.1046/j.1460-9568.2000.00996.x.
- ↑ Akiyama, Gaku; Ikeda, Hiroko; Matsuzaki, Satoshi; Sato, Michiko; Moribe, Shoko; Koshikawa, Noriaki; Cools, Alexander R. (2004). "GABAA and GABAB receptors in the nucleus accumbens shell differentially modulate dopamine and acetylcholine receptor-mediated turning behaviour". Neuropharmacology. 46 (8): 1082–8. PMID 15111014. doi:10.1016/j.neuropharm.2004.02.007.
- ↑ Smith-Roe, Stephanie L.; Sadeghian, Kenneth; Kelley, Ann E. (1999). "Spatial learning and performance in the radial arm maze is impaired after N-methyl-d-aspartate (NMDA) receptor blockade in striatal subregions". Behavioral Neuroscience. 113 (4): 703–17. PMID 10495079. doi:10.1037/0735-7044.113.4.703.
- ↑ Giertler, Christian; Bohn, Ines; Hauber, Wolfgang (2005). "Involvement of NMDA and AMPA/KA receptors in the nucleus accumbens core in instrumental learning guided by reward-predictive cues". European Journal of Neuroscience. 21 (6): 1689–702. PMID 15845096. doi:10.1111/j.1460-9568.2005.03983.x.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Olsen, Christopher M. (2011). "Natural rewards, neuroplasticity, and non-drug addictions". Neuropharmacology. 61 (7): 1109–22. PMC 3139704
 . PMID 21459101. doi:10.1016/j.neuropharm.2011.03.010. Retrieved 10 September 2014.
. PMID 21459101. doi:10.1016/j.neuropharm.2011.03.010. Retrieved 10 September 2014. Cross-sensitization is also bidirectional, as a history of amphetamine administration facilitates sexual behavior and enhances the associated increase in NAc DA ... As described for food reward, sexual experience can also lead to activation of plasticity-related signaling cascades. The transcription factor delta FosB is increased in the NAc, PFC, dorsal striatum, and VTA following repeated sexual behavior (Wallace et al., 2008; Pitchers et al., 2010b). This natural increase in delta FosB or viral overexpression of delta FosB within the NAc modulates sexual performance, and NAc blockade of delta FosB attenuates this behavior (Hedges et al, 2009; Pitchers et al., 2010b). Further, viral overexpression of delta FosB enhances the conditioned place preference for an environment paired with sexual experience (Hedges et al., 2009). ...
Table 1 - ↑ Costa, Vincent D.; Lang, Peter J.; Sabatinelli, Dean; Versace, Francesco; Bradley, Margaret M. (2010). "Emotional imagery: Assessing pleasure and arousal in the brain's reward circuitry". Human Brain Mapping. 31 (9): 1446–57. PMC 3620013
 . PMID 20127869. doi:10.1002/hbm.20948.
. PMID 20127869. doi:10.1002/hbm.20948. - ↑ Sabatinelli, D.; Bradley, M. M.; Lang, P. J.; Costa, V. D.; Versace, F. (2007). "Pleasure Rather Than Salience Activates Human Nucleus Accumbens and Medial Prefrontal Cortex". Journal of Neurophysiology. 98 (3): 1374–9. PMID 17596422. doi:10.1152/jn.00230.2007.
- ↑ Menon, V.; Levitin, D.J. (2005). "The rewards of music listening: Response and physiological connectivity of the mesolimbic system". NeuroImage. 28 (1): 175–84. PMID 16023376. doi:10.1016/j.neuroimage.2005.05.053.
- ↑ Olds, James; Milner, Peter (1954). "Positive Reinforcement Produced by Electrical Stimulation of Septal Area and Other Regions of Rat Brain". Journal of Comparative and Physiological Psychology. 47 (6): 419–27. PMID 13233369. doi:10.1037/h0058775.
- ↑ Nakahara, Daiichiro; Ozaki, Norio; Miura, Yoshihiro; Miura, Hideki; Nagatsu, Toshiharu (1989). "Increased dopamine and serotonin metabolism in rat nucleus accumbens produced by intracranial self-stimulation of medial forebrain bundle as measured by in vivo microdialysis". Brain Research. 495 (1): 178–81. PMID 2476201. doi:10.1016/0006-8993(89)91234-1.
- ↑ Aharon, Itzhak; Etcoff, Nancy; Ariely, Dan; Chabris, Christopher F; O'Connor, Ethan; Breiter, Hans C (2001). "Beautiful Faces Have Variable Reward Value". Neuron. 32 (3): 537–51. PMID 11709163. doi:10.1016/S0896-6273(01)00491-3.
- ↑ Ferris, C. F.; Kulkarni, P; Sullivan Jr, J. M.; Harder, J. A.; Messenger, T. L.; Febo, M (2005). "Pup Suckling is More Rewarding Than Cocaine: Evidence from Functional Magnetic Resonance Imaging and Three-Dimensional Computational Analysis". Journal of Neuroscience. 25 (1): 149–56. PMID 15634776. doi:10.1523/jneurosci.3156-04.2005.
- ↑ Numan, Michael (2007). "Motivational systems and the neural circuitry of maternal behavior in the rat". Developmental Psychobiology. 49 (1): 12–21. PMID 17186513. doi:10.1002/dev.20198.
- ↑ Glocker, M. L.; Langleben, D. D.; Ruparel, K.; Loughead, J. W.; Valdez, J. N.; Griffin, M. D.; Sachser, N.; Gur, R. C. (2009). "Baby schema modulates the brain reward system in nulliparous women". Proceedings of the National Academy of Sciences. 106 (22): 9115–9. Bibcode:2009PNAS..106.9115G. JSTOR 40482823. PMC 2690007
 . PMID 19451625. doi:10.1073/pnas.0811620106.
. PMID 19451625. doi:10.1073/pnas.0811620106. - 1 2 Hyman, Steven E.; Malenka, Robert C.; Nestler, Eric J. (2006). "Neural mechanisms of addiction: the role of reward-related learning and memory". Annual Review of Neuroscience. 29: 565–98. PMID 16776597. doi:10.1146/annurev.neuro.29.051605.113009.
- ↑ Steiner, Heinz; Van Waes, Vincent (2013). "Addiction-related gene regulation: Risks of exposure to cognitive enhancers vs. Other psychostimulants". Progress in Neurobiology. 100: 60–80. PMC 3525776
 . PMID 23085425. doi:10.1016/j.pneurobio.2012.10.001.
. PMID 23085425. doi:10.1016/j.pneurobio.2012.10.001. - 1 2 Ruffle JK (Nov 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". The American Journal of Drug and Alcohol Abuse. 40 (6): 428–37. PMID 25083822. doi:10.3109/00952990.2014.933840.
ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure. The formation of ΔFosB in multiple brain regions, and the molecular pathway leading to the formation of AP-1 complexes is well understood. The establishment of a functional purpose for ΔFosB has allowed further determination as to some of the key aspects of its molecular cascades, involving effectors such as GluR2 (87,88), Cdk5 (93) and NFkB (100). Moreover, many of these molecular changes identified are now directly linked to the structural, physiological and behavioral changes observed following chronic drug exposure (60,95,97,102). New frontiers of research investigating the molecular roles of ΔFosB have been opened by epigenetic studies, and recent advances have illustrated the role of ΔFosB acting on DNA and histones, truly as a ‘‘molecular switch’’ (34).
- ↑ Kanehisa Laboratories (29 October 2014). "Alcoholism – Homo sapiens (human)". KEGG Pathway. Retrieved 31 October 2014.
- ↑ Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P (Feb 2009). "Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens". Proceedings of the National Academy of Sciences of the United States of America. 106 (8): 2915–20. PMC 2650365
 . PMID 19202072. doi:10.1073/pnas.0813179106.
. PMID 19202072. doi:10.1073/pnas.0813179106. - ↑ Pitchers, K. K.; Vialou, V.; Nestler, E. J.; Laviolette, S. R.; Lehman, M. N.; Coolen, L. M. (2013). "Natural and Drug Rewards Act on Common Neural Plasticity Mechanisms with FosB as a Key Mediator". Journal of Neuroscience. 33 (8): 3434–42. PMC 3865508
 . PMID 23426671. doi:10.1523/JNEUROSCI.4881-12.2013.
. PMID 23426671. doi:10.1523/JNEUROSCI.4881-12.2013. Drugs of abuse induce neuroplasticity in the natural reward pathway, specifically the nucleus accumbens (NAc), thereby causing development and expression of addictive behavior. ... Together, these findings demonstrate that drugs of abuse and natural reward behaviors act on common molecular and cellular mechanisms of plasticity that control vulnerability to drug addiction, and that this increased vulnerability is mediated by ΔFosB and its downstream transcriptional targets. ... Sexual behavior is highly rewarding (Tenk et al., 2009), and sexual experience causes sensitized drug-related behaviors, including cross-sensitization to amphetamine (Amph)-induced locomotor activity (Bradley and Meisel, 2001; Pitchers et al., 2010a) and enhanced Amph reward (Pitchers et al., 2010a). Moreover, sexual experience induces neural plasticity in the NAc similar to that induced by psychostimulant exposure, including increased dendritic spine density (Meisel and Mullins, 2006; Pitchers et al., 2010a), altered glutamate receptor trafficking, and decreased synaptic strength in prefrontal cortex-responding NAc shell neurons (Pitchers et al., 2012). Finally, periods of abstinence from sexual experience were found to be critical for enhanced Amph reward, NAc spinogenesis (Pitchers et al., 2010a), and glutamate receptor trafficking (Pitchers et al., 2012). These findings suggest that natural and drug reward experiences share common mechanisms of neural plasticity
- ↑ Brain Electrodes Help Treat Depression, Technology Review, 26 April 2007
- ↑ Bewernick, Bettina H.; Hurlemann, René; Matusch, Andreas; Kayser, Sarah; Grubert, Christiane; Hadrysiewicz, Barbara; Axmacher, Nikolai; Lemke, Matthias; Cooper-Mahkorn, Deirdre; Cohen, Michael X.; Brockmann, Holger; Lenartz, Doris; Sturm, Volker; Schlaepfer, Thomas E. (2010). "Nucleus Accumbens Deep Brain Stimulation Decreases Ratings of Depression and Anxiety in Treatment-Resistant Depression". Biological Psychiatry. 67 (2): 110–6. PMID 19914605. doi:10.1016/j.biopsych.2009.09.013.
- ↑ Ooms, P.; Mantione, M.; Figee, M.; Schuurman, P. R.; Van Den Munckhof, P.; Denys, D. (2013). "Deep brain stimulation for obsessive-compulsive disorders: Long-term analysis of quality of life". Journal of Neurology, Neurosurgery & Psychiatry. 85 (2): 153–8. PMID 23715912. doi:10.1136/jnnp-2012-302550.
- ↑ "Controversial Surgery for Addiction Burns Away Brain’s Pleasure Center" Author Maia Szalavitz. Dec. 13, 2012
- ↑ "China Bans Irreversible Brain Procedure" Author Zamiska Nicholas. April 28, 2008. The Wall Street Journal
- ↑ Scott, David J.; Stohler, Christian S.; Egnatuk, Christine M.; Wang, Heng; Koeppe, Robert A.; Zubieta, Jon-Kar (2007). "Individual Differences in Reward Responding Explain Placebo-Induced Expectations and Effects". Neuron. 55 (2): 325–36. PMID 17640532. doi:10.1016/j.neuron.2007.06.028. Lay summary – Cell Press (July 18, 2007).
External links
| Wikimedia Commons has media related to Nucleus accumbens. |
- The role of the nucleus accumbens in the reward circuit. Part of "The Brain From Top to Bottom." at thebrain.mcgill.ca
- Nucleus Accumbens – Cell Centered Database
- Stained brain slice images which include the "nucleus%20accumbens" at the BrainMaps project