Nonmetal
| Part of a series on the |
| Periodic table |
|---|
|
By other characteristics: |
|
|
|
Data pages for elements
|
|
In chemistry, a nonmetal (or non-metal) is a chemical element that mostly lacks metallic attributes. Physically, nonmetals tend to be highly volatile (easily vaporized), have low elasticity, and are good insulators of heat and electricity; chemically, they tend to have high ionization energy and electronegativity values, and gain or share electrons when they react with other elements or compounds. Seventeen elements are generally classified as nonmetals; most are gases (hydrogen, helium, nitrogen, oxygen, fluorine, neon, chlorine, argon, krypton, xenon and radon); one is a liquid (bromine), and a few are solids (carbon, phosphorus, sulphur, selenium, and iodine).
Moving rightward across the standard form of the periodic table, nonmetals adopt structures that have progressively fewer nearest neighbours. Polyatomic nonmetals have structures with either three nearest neighbours, as is the case (for example) with carbon (in its standard state[n 1] of graphite), or two nearest neighbours (for example) in the case of sulfur. Diatomic nonmetals, such as hydrogen, have one nearest neighbour, and the monatomic noble gases, such as helium, have none. This gradual fall in the number of nearest neighbours is associated with a reduction in metallic character and an increase in nonmetallic character. The distinction between the three categories of nonmetals, in terms of receding metallicity is not absolute. Boundary overlaps occur as outlying elements in each category show (or begin to show) less-distinct, hybrid-like or atypical properties.
Although five times more elements are metals than nonmetals, two of the nonmetals—hydrogen and helium—make up over 99 per cent of the observable Universe,[4] and one—oxygen—makes up close to half of the Earth's crust, oceans and atmosphere.[5] Living organisms are also composed almost entirely of nonmetals,[6] and nonmetals form many more compounds than metals.[7]
Definition and properties
JJ Zuckerman and FC Nachod
In Steudel's Chemistry of the non-metals (1977, preface)
There is no rigorous definition of a nonmetal. They show more variability in their properties than metals do.[8] The following are some of the chief characteristics of nonmetals.[9] Physically, they largely exist as monatomic gases, with a few having more substantial (but still open-packed) diatomic or polyatomic forms, unlike metals, which are nearly all solid and close-packed; if solid, they generally have a submetallic or dull appearance and are brittle, as opposed to metals, which are lustrous, ductile or malleable; they usually have lower densities than metals; are poor conductors of heat and electricity when compared to metals; and have significantly lower melting points and boiling points than those of metals (with the exception of carbon). Chemically, the nonmetals have relatively high ionisation energy and high electronegativity; they usually exist as anions or oxyanions in aqueous solution; generally form ionic or interstitial compounds when mixed with metals, unlike metals, which form alloys; and have acidic oxides whereas the common oxides of the metals are basic.
Applicable elements
| Nonmetals in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arbitrary set (see text) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The elements generally classified as nonmetals include one element in group 1 (hydrogen); one in group 14 (carbon); two in group 15 (nitrogen and phosphorus); three in group 16 (oxygen, sulfur and selenium); most of group 17 (fluorine, chlorine, bromine and iodine); and all of group 18 (with the possible exception of oganesson).
The distinction between nonmetals and metals is by no means clear.[13] The result is that a few borderline elements lacking a preponderance of either nonmetallic or metallic properties are classified as metalloids;[14] and some elements classified as nonmetals are instead sometimes classified as metalloids, or vice versa. For example, selenium (Se), a nonmetal, is sometimes classified instead as a metalloid, particularly in environmental chemistry;[15] and astatine (At), which is a metalloid and a halogen, is sometimes classified instead as a nonmetal.[16]
Categories
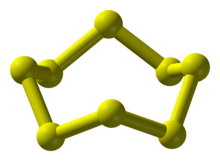
Nonmetals have structures in which each atom usually forms (8 − N) bonds with (8 − N) nearest neighbours, where N is the number of valence electrons. Each atom is thereby able to complete its valence shell and attain a stable noble gas configuration. Exceptions to the (8 − N) rule occur with hydrogen (which only needs one bond to complete its valence shell), carbon, nitrogen and oxygen. Atoms of the latter three elements are sufficiently small such that they are able to form alternative (more stable) bonding structures, with fewer nearest neighbours.[17] Thus, carbon is able to form its layered graphite structure, and nitrogen and oxygen are able to form diatomic molecules having triple and double bonds, respectively. The larger size of the remaining non-noble nonmetals weakens their capacity to form multiple bonds and they instead form two or more single bonds to two or more different atoms.[18] Sulfur, for example, forms an eight-membered molecule in which the atoms are arranged in a ring, with each atom forming two single bonds to different atoms.

From left to right across the standard form of periodic table, as metallic character decreases,[21] nonmetals therefore adopt structures that show a gradual reduction in the numbers of nearest neighbours—three or two for the polyatomic nonmetals, through one for the diatomic nonmetals, to zero for the monatomic noble gases. A similar pattern occurs more generally, at the level of the entire periodic table, in comparing metals and nonmetals. There is a transition from metallic bonding among the metals on the left of the table through to covalent or Van der Waals (electrostatic) bonding among the nonmetals on the right of the table.[22] Metallic bonding tends to involve close-packed centrosymmetric structures with a high number of nearest neighbours.[23] Post-transition metals and metalloids, sandwiched between the true metals[n 3] and the nonmetals, tend to have more complex structures with an intermediate number of nearest neighbours.[n 4] Nonmetallic bonding, towards the right of the table, features open-packed directional (or disordered) structures with fewer or zero nearest neighbours.[26] As noted, this steady reduction in the number of nearest neighbours, as metallic character decreases and nonmetallic character increases, is mirrored among the nonmetals, the structures of which gradually change from polyatomic, to diatomic, to monatomic.
As is the case with the major categories of metals, metalloids and nonmetals,[27] there is some variation and overlapping of properties within and across each category of nonmetal. Among the polyatomic nonmetals, carbon, phosphorus and selenium—which border the metalloids—begin to show some metallic character. Sulfur (which borders the diatomic nonmetals), is the least metallic of the polyatomic nonmetals but even here shows some discernible metal-like character (discussed below). Of the diatomic nonmetals, iodine is the most metallic. Its number of nearest neighbours is sometimes described as 1+2 hence it is almost a polyatomic nonmetal.[28] Within the iodine molecule, significant electronic interactions occur with the two next nearest neighbours of each atom, and these interactions give rise, in bulk iodine, to a shiny appearance and semiconducting properties.[29] Of the monatomic nonmetals, radon is the most metallic and begins to show some cationic behaviour, which is unusual for a nonmetal.[30]
Polyatomic nonmetals

| Polyatomic nonmetals in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Four nonmetals are distinguished by polyatomic bonding in their standard states, in either discrete or extended molecular forms: carbon (C, as graphite sheets); phosphorus (as P4 molecules); sulfur (as S8 molecules); and selenium (Se, as helical chains).[32] Consistent with their higher coordination numbers (2 or 3), the polyatomic nonmetals show more metallic character than the neighbouring diatomic nonmetals; they are all solid, mostly semi-lustrous semiconductors with electronegativity values that are intermediate to moderately high (2.19–2.58). Sulfur is the least metallic of the polyatomic nonmetals given its dull appearance, brittle comportment, and low conductivity—attributes common to all sulfur allotropes. It nevertheless shows some metallic character, either intrinsically or in its compounds with other nonmetals. Examples include the malleability of plastic sulfur[33] and the lustrous-bronze appearance and metallic conductivity of polysulfur nitride (SNx).[34][n 5]
The polyatomic nonmetals are distinguished from the diatomic nonmetals by virtue of having higher coordination numbers, higher melting points (in their thermodynamically most stable forms), and higher boiling points; and having wider liquid ranges and lower room temperature volatility.[56] More generally they show a marked tendency to exist in allotropic forms, and a stronger inclination to catenate;[57] and have a weaker ability to form hydrogen bonds.[58] The ability of carbon to catenate, in particular, is fundamental to the field of organic chemistry and life on Earth.[59][n 6] All of the polyatomic nonmetals are solids, and all are known in either malleable, pliable or ductile forms; most also have lower ionisation energies and electronegativities than those of the diatomic nonmetals.
Diatomic nonmetals
| Diatomic nonmetals in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Seven nonmetals exist as diatomic molecules in their standard states: hydrogen (H2); nitrogen (N2); oxygen (O2); fluorine (F2); chlorine (Cl2); bromine (Br2); and iodine (I2).[61] They are generally highly insulating, highly electronegative, non-reflective gases, noting that bromine, a liquid, and iodine, a solid, are both volatile at room temperature.[62][63] Exceptions to this generalised description occur at the boundaries of the category: hydrogen has a comparatively low electronegativity due to its unique atomic structure;[n 7] iodine, in crystalline form, is semi-lustrous, and a semiconductor in the direction of its layers,[65][n 8] both of these attributes being consistent with incipient metallic character.
The diatomic nonmetals are distinguished from the polyatomic nonmetals by virtue of having lower coordination numbers, lower melting points (compared to the polyatomic nonmetals in their thermodynamically most stable forms), and lower boiling points; and having narrower liquid ranges[n 9] and greater room temperature volatility. More generally, they show less inclination to exist in allotropic forms, and to catenate; and have a stronger ability to form hydrogen bonds. Most are also gases, and have higher ionisation energies and higher electronegativities than those of the polyatomic nonmetals.
Noble gases

| Noble gases in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Six nonmetals occur naturally as monatomic noble gases: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn). They comprise a group of chemical elements with very similar properties. In their standard states they are all colorless, odourless, nonflammable gases with characteristically very low chemical reactivity.
With their closed valence shells, the noble gases have the highest first ionization potentials in each of their periods, and feeble interatomic forces of attraction, with the latter property resulting in very low melting and boiling points.[68] That is why they are all gases under standard conditions, even those with atomic masses larger than many normally solid elements.[69]
The status of the period 7 congener of the noble gases, oganesson (Og), is not known—it may or may not be a noble gas. It was originally predicted to be a noble gas[70] but may instead be a fairly reactive solid with an anomalously low first ionisation potential, due to relativistic effects.[71] On the other hand, if relativistic effects peak in period 7 at element 112, copernicium (as is thought to be the case), oganesson may turn out to be a noble gas after all,[72] albeit more reactive than either xenon or radon. Regardless, oganesson is predicted to be a nonmetal, and probably the only one in period 7.
Elemental gases
| Elemental gases in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hydrogen, nitrogen, oxygen, fluorine, chlorine, plus the noble gases are collectively referred to as the elemental gases. These elements are gaseous at standard temperature and pressure (STP). They are also distinguished by having the lowest densities, lowest melting and boiling points, strongest insulating properties, and highest electronegativity and ionization energy values in the periodic table.
It is not known if any synthetic elements with atomic number above 99 are gases. If it transpires that copernicium and flerovium are gaseous metals at or near room temperature, as some calculations as well as preliminary experimental results have suggested,[73] the category of elemental gases may need to be sub-divided into metallic and nonmetallic gases.
Organogens, CHONPS and biogens
Carbon, hydrogen, oxygen, nitrogen, phosphorus and sulfur are sometimes referred to or categorised as organogens,[74] CHONPS elements[75] or biogens.[76][n 10] Collectively these six nonmetals are required for all life on Earth.[77][n 11] They are further distinguished—in comparison to the halogens (F, Cl, Br, I, At) and noble gases—by their general capacity (or potential) to form allotropes;[n 12] high atomisation energies;[84] intermediate electron affinities;[n 13] reactivity combined with low toxicity;[n 14] ability to form alloys with metals;[87] and the weak or neutral acid-base character of their group hydrides.[88]
Other nonmetals
Selenium, and possibly boron, silicon, arsenic and tellurium, plus the organogen elements are sometimes categorized together as other nonmetals, as they fall outside the halogens and noble gases.[89] The first five of these (Se; B, Si, As, Te) differ from the organogens: none are universally required for life; arsenic is notoriously poisonous; and tellurium hydride is a fairly strong, rather than weak, acidic hydride.[90]
Comparison of properties
Characteristic and other properties of polyatomic nonmetals, diatomic nonmetals, and the monatomic noble gases are summarized in the following table. Physical properties are listed in loose order of ease of determination; chemical properties run from general to specific, and then to descriptive.
| Physical property | Polyatomic nonmetals | Diatomic nonmetals | Monatomic noble gases |
|---|---|---|---|
| Form | solid | mostly gaseous | gaseous |
| Appearance | most are lustrous | several colourless; others dull red, yellow, green or intermediate shades; iodine is grey or bluish-black and shows some lustre | colourless |
| Bulk coordination number | 2–3 (diamond: 4) | 1 | 0 |
| Allotropy | marked tendency | less inclination | nil |
| Elasticity | most are brittle; all are known in malleable (C), pliable (P) or ductile (C, S, Se) forms[n 15] | brittle if solid | soft and easily crushed when frozen[95] |
| Electrical conductivity (S•cm−1) | poor to good (from 5.2 × 10−18 for S[96] to 3 × 104 for graphitic C)[97] | poor to low (from ~10−18 for the diatomic gases[98] to 1.7 × 10−8 for iodine)[99] | poor (~10−18)[98] |
| Melting point (K) | higher (389–3,800) | lower (15–387) | mostly lowest (1–202) |
| Boiling point | higher (718–4,300) | lower (21–458) | mostly lowest (5–212) |
| Liquid range (K) | relatively narrow (232–505) | narrower still (6–70) | mostly narrowest (2–9) |
| Volatility (room temperature) | lower | higher | highest (on average)[56] |
| Chemical property | Polyatomic nonmetals | Diatomic nonmetals | Monatomic noble gases |
| General chemical behaviour | nonmetallic to incipient metallic | nonmetallic; iodine shows incipient metallic behaviour | inert to nonmetallic; Rn shows some cationic behaviour[100] |
| Ionization energy (kJ•mol−1) | mostly lower (9.75–11.26) | mostly higher (10.45–17.42) | mostly highest (10.75–24.59) |
| Electronegativity (Allen scale) | mostly lower (2.253–2.589) | mostly higher (2.300–4.193) | mostly highest (2.582–4.789) |
| Oxidation states | • negative and positive oxidation states known for all • from ‒4 for C to +6 for S and Se |
• negative oxidation states known for all, but for H this is an unstable state • positive oxidation states known for all bar F, and only exceptionally for O • from ‒3 for N to +7 for Cl, Br and I |
• only positive oxidation states known, and only for heavier noble gases • from +2 for Kr, Xe, and Rn to +8 for Xe |
| Catenation | marked tendency | less inclination | least inclination |
| Hydrogen bonds | weaker ability | stronger ability | known for Ar, Kr, Xe[101] |
| Oxides | • all known in at least one polymeric form • most (P, S, Se) are glass formers; CO2 forms a glass at 40 GPa |
• iodine oxides known in polymeric forms[102] • no known glass formers |
• XeO2 is polymeric;[103] other noble gas oxides are molecular • no glass formers |
Allotropes
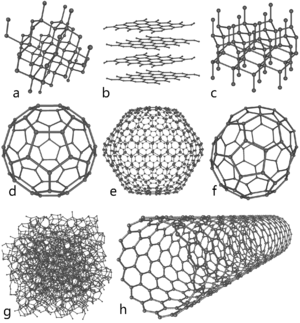
Many nonmetals have less stable allotropes, with either nonmetallic or metallic properties. Graphite, the standard state of carbon, has a lustrous appearance and is a fairly good electrical conductor. The diamond allotrope of carbon is clearly nonmetallic, however, being translucent and having a relatively poor electrical conductivity. Carbon is also known in several other allotropic forms, including semiconducting buckminsterfullerene (C60). Nitrogen can form gaseous tetranitrogen (N4), an unstable polyatomic molecule with a lifetime of about one microsecond.[104] Oxygen is a diatomic molecule in its standard state; it also exists as ozone (O3), an unstable polyatomic nonmetallic allotrope with a half-life of around half an hour.[105] Phosphorus, uniquely, exists in several allotropic forms that are more stable than that of its standard state as white phosphorus (P4).[n 16] The red and black allotropes are probably the best known; both are semiconductors; black phosphorus, in addition, has a lustrous appearance. Phosphorus is also known as diphosphorus (P2), an unstable diatomic allotrope.[106] Sulfur has more allotropes than any other element;[107] all of these, except plastic sulfur (a metastable ductile mixture of allotropes)[108] have nonmetallic properties. Selenium has several nonmetallic allotropes, all of which are much less electrically conducting than its standard state of grey "metallic" selenium.[109] Iodine is also known in a semiconducting amorphous form.[110] Under sufficiently high pressures, just over half of the nonmetals, starting with phosphorus at 1.7 GPa,[111] have been observed to form metallic allotropes.
Abundance and extraction

Hydrogen and helium are estimated to make up approximately 99 per cent of all ordinary matter in the universe. Less than five per cent of the Universe is believed to be made of ordinary matter, represented by stars, planets and living beings. The balance is made of dark energy and dark matter, both of which are poorly understood at present.[112]
Hydrogen, carbon, nitrogen, and oxygen constitute the great bulk of the Earth's atmosphere, oceans, crust, and biosphere; the remaining nonmetals have abundances of 0.5 per cent or less. In comparison, 35 per cent of the crust is made up of the metals sodium, magnesium, aluminium, potassium and iron; together with a metalloid, silicon. All other metals and metalloids have abundances within the crust, oceans or biosphere of 0.2 per cent or less.[113]
Nonmetals, in their elemental forms, are extracted from:[114] brine: Cl, Br, I; liquid air: N, O, Ne, Ar, Kr, Xe; minerals: C (coal; diamond; graphite); F (fluorite); P (phosphates); I (in sodium iodate NaIO3 and sodium iodide NaI); natural gas: H, He, S; and from ores, as processing byproducts: Se (especially copper ores); and Rn (uranium bearing ores).
Applications in common
- For prevalent and speciality applications of individual nonmetals see the main article for each element.

Nonmetals do not have any universal or near-universal applications. This is not the case with metals, most of which have structural uses; nor the metalloids, the typical uses of which extend to (for example) oxide glasses, alloying components, and semiconductors.
Shared applications of different subsets of the nonmetals instead encompass their presence in, or specific uses in the fields of cryogenics and refrigerants: H, He, N, O, F and Ne; fertilisers: H, N, P, S, Cl (as a micronutrient) and Se; household accoutrements: H (primary constituent of water), He (party balloons), C (in pencils, as graphite), N (beer widgets), O (as peroxide, in detergents), F (as fluoride, in toothpaste), Ne (lighting), P (matches), S (garden treatments), Cl (bleach constituent), Ar (insulated windows), Se (glass; solar cells), Br (as bromide, for purification of spa water), Kr (energy saving fluorescent lamps), I (in antiseptic solutions), Xe (in plasma TV display cells), while Rn also sometimes occurs, but then as an unwanted, potentially hazardous indoor pollutant;[116] industrial acids: C, N, F, P, S and Cl; inert air replacements: N, Ne, S (in sulfur hexafluoride SF6), Ar, Kr and Xe; lasers and lighting: He, C (in carbon dioxide lasers, CO2), N, O (in a chemical oxygen iodine laser), F (in a hydrogen fluoride laser, HF), Ne, S (in a sulfur lamp), Ar, Kr and Xe; and medicine and pharmaceuticals: He, O, F, Cl, Br, I, Xe and Rn.
The number of compounds formed by nonmetals is vast.[117] The first nine places in a "top 20" table of elements most frequently encountered in 8,427,300 compounds, as listed in the Chemical Abstracts Service register for July 1987, were occupied by nonmetals. Hydrogen, carbon, oxygen and nitrogen were found in the majority (greater than 64 per cent) of compounds. The highest rated metal, with an occurrence frequency of 2.3 per cent, was iron, in 11th place.[118]
Discovery
Antiquity: C, S
Sulfur and carbon were known in antiquity. The earliest known use of charcoal dates to around 3750 BCE. The Egyptians and Sumerians employed it for the reduction of copper, zinc, and tin ores in the manufacture of bronze. Diamonds were probably known from as early as 2500 BCE. The first true chemical analyses were made in the 18th century; Lavoisier recognized carbon as an element in 1789. Sulfur usage dates from before 2500 BCE; it was recognized as an element by Antoine Lavoisier in 1777.
17th century: P
Phosphorus was prepared from urine, by Hennig Brand, in 1669. It was the first element to be chemically discovered.
18th century: H, O, N, Cl
Cavendish, in 1766, was the first to distinguish hydrogen from other gases, although Paracelsus around 1500, Robert Boyle (1670), and Joseph Priestley (?) had observed its production by reacting strong acids with metals. Lavoisier named it in 1793. Carl Wilhelm Scheele obtained oxygen by heating mercuric oxide and nitrates in 1771, but did not publish his findings until 1777. Priestley also prepared this new "air" by 1774, but only Lavoisier recognized it as a true element; he named it in 1777. Daniel Rutherford discovered nitrogen while he was studying at the University of Edinburgh. He showed that the air in which animals breathed, after removal of exhaled carbon dioxide, was no longer able to burn a candle. Scheele, Henry Cavendish, and Priestley also studied this element at about the same time; Lavoisier named it in 1775-6. Scheele obtained chlorine from hydrochloric acid, but thought it was an oxide. Only in 1808 did Humphry Davy recognize it as an element.
Early 19th century: I, Se, Br
Courtois, in 1811, discovered iodine in the ashes of seaweed. In 1817, when Berzelius and Johan Gottlieb Gahn were working with lead they discovered a substance that was similar to tellurium. After more investigation Berzelius concluded that it was a new element, related to sulfur and tellurium. Because tellurium had been named for the Earth, Berzelius named the new element "selenium", after the moon. Balard and Gmelin both discovered bromine in the autumn of 1825 and published their results in the following year.
Late 19th century: He, F, Ar, Kr, Ne, Xe, Rn
In 1868, Janssen and Lockyer independently observed a yellow line in the solar spectrum that did not match that of any other element. In 1895, in each case at around the same time, Ramsay, Cleve, and Langlet independently observed helium trapped in cleveite. André-Marie Ampère predicted an element analogous to chlorine obtainable from hydrofluoric acid, and between 1812 and 1886 many researchers tried to obtain it. Fluorine was eventually isolated by Moissan, in 1886. In 1894, Lord Rayleigh and Ramsay discovered argon by comparing the molecular weights of nitrogen prepared by liquefaction from air and nitrogen prepared by chemical means. It was the first noble gas to be isolated. In 1898, within a period of three weeks, Ramsay and Travers successively separated krypton, neon and xenon from liquid argon by their differences in boiling points. In 1898, Dorn discovered a radioactive gas resulting from the radioactive decay of radium; Ramsay and Robert Whytlaw-Gray subsequently isolated radon in 1910.
Notes
- ↑ The standard state of an element (with one exception) is the most thermodynamically stable form of the element at ambient conditions. The exception is phosphorus, the standard state of which is the white allotrope, the most thermodynamically unstable, as well as the most volatile and reactive form.[1] It is also the most common, industrially important,[2] and easily reproducible allotrope. For those reasons white phosphorus (rather than the black allotrope, which is the most thermodynamically stable form) is the standard state of the element.[3]
- ↑ Triple bonding is thought to represent the limit for main-group elements.[19] Of the diatomic nonmetals only hydrogen has a shorter bond length, at 74 pm.[20]
- ↑ True metals are the alkali metals, alkaline earth metals, lanthanides, actinides, and d-block metals up to group 11.[24]
- ↑ Structural complexity in the post-transition metals arises due to the influence of partially covalent bonding, the directionality of which dictates fewer nearest neighbours.[25]
- ↑ Metallic or metalloidal character is further shown by sulfur, as follows. It is a solid, as are nearly all metals, and all metalloids, whereas the large majority of nonmetals are not. It has the highest superconducting transition phase critical temperature (10 K at 93 GPa) among the non-metallic elements.[35] Its most stable oxidation state is +6.[36] It has a well-established cationic chemistry in superacidic media, extending to the isolation of sulfur salts such as [S4]2+[SbF6]−2, a pale-yellow solid that is stable at room temperature.[37][38] The lower electronegativity of sulfur compared to its lighter congenor, oxygen, largely means that transition metal sulfides are more likely to be alloy-like semiconductors or metallic conductors than the corresponding oxides,[39] a trend also evident in lanthanide sulfides. Thus, cerium sulfide (CeS) has a metallic bronze lustre, exhibits high-level metallic electrical conductivity, and can be machined like a metal,[40][41] and samarium sulfide (SmS), a black semiconductor, can reportedly adopt a golden metallic phase by way of pressure, polishing or simply scratching on single crystals.[42] The diminished nonmetallic character of sulfur (and conversely its increased metallic character) is further shown by the fact that zinc sulfide does not dissolve in alkaline solution whereas zinc oxide does.[43] Sulfur can be combined with carbon to form a disulfide (CS2), which, above 50 GPa, undergoes an insulator-to-metal transition. As yet this is puzzling behaviour given the most "metallic" organic polymers, other than compressed CS2, exhibit barely metallic conductivity.[44] When sulfur is introduced as a doping agent, it causes silicon to exhibit metal-like conduction and associated enhanced light absorption characteristics (from low-frequency visible light through near- and short-wave-infrared wavelengths that would normally pass right through regular silicon).[45][46] Sulfur is a photoconductor (sometimes described as a semiconductor),[47] which means that its electrical conductivity increases by up to a million-fold when illuminated.[48] The first photocopy was produced in 1938 using a photoconductive layer of fused sulfur on a zinc plate.[49] Sulfur becomes a (liquid) semiconductor at 900 °C, with an electrical conductivity of 5 × 10−5 S•cm−1 (about a trillion times that of its room temperature conductivity, and twice that of boron, a metalloid).[50][51] In 2013 researchers reported metallic conductivity in linear chains of sulfur atoms, isolated inside carbon nanotubes, at ambient conditions.[52] Sulfur trioxide is a glass-former,[53] as are oxides of phosphorus and selenium[54] and, at 40 GPa, carbon dioxide.[55]
- ↑ After carbon, phosphorus shows the next strongest ability to catenate.[60]
- ↑ Hydrogen's single electron is not shielded from the single proton in its nucleus, resulting in hydrogen having an ionisation energy on par with that of oxygen. However, this configuration also means that a hydrogen atom's ability to attract another electron to itself is compromised by its single proton not being able to fully stabilise the electron-electron repulsion forces that arise between two valence shell electrons (resulting in the instability of the hydride H− ion).[64]
- ↑ Iodine is an insulator in the direction perpendicular to its crystalline layers.[66]
- ↑ The liquid range of an element is the difference between its melting point and boiling point.[67]
- ↑ Selenium is counted as a metalloid in the last case, rather than a nonmetal.[76]
- ↑ The same elements comprise the bulk of terrestrial life;[78] a rough estimate[79] of the composition of the biosphere is C1450H3000O1450N15P1S1
- ↑ Carbon, oxygen, phosphorus and sulfur exist in well known allotropic forms. Hydrogen is known in metastable monatomic and unstable triatomic forms, in addition to its stable diatomic form. Monatomic hydrogen has a lifetime of a few tenths of a second to hours depending on the production technique (heating, electric arc or discharge, UV or microwave irradiation, electron bombardment) and containment method.[80] It can be used, via a Langmuir torch, to weld or melt high melting point metals or compounds.[81] Triatomic hydrogen is unstable and breaks up in under a millionth of a second. Its fleeting lifetime makes it rare, but it is quite commonly formed and destroyed in the universe.[82] For nitrogen, the as yet unsynthesized allotrope octaazacubane (N8) is predicted to be metastable.[83]
- ↑ Electron affinities of the CHNOPS elements are –0.07 to +2.08 eV; halogens +2.8 to +3.4; noble gases –0.08 to –0.42[85]
- ↑ Phosphorus in its most thermodynamically stable black form is generally inert (although still reactive compared to the noble gases) and has a low toxicity.[86] White phosphorus, the most commonly known and encountered form, is metastable, highly reactive, flammable and poisonous.
- ↑ Carbon as exfoliated (expanded) graphite,[91] and as metre-long carbon nanotube wire;[92] phosphorus as white phosphorus (soft as wax, pliable and can be cut with a knife, at room temperature);[93] sulfur as plastic sulfur;[33] and selenium as selenium wires.[94]
- ↑ White phosphorus is the most common, industrially important,[2] and easily reproducible allotrope. For those reasons it is the standard state of the element.[3] Paradoxically, it is also thermodynamically the least stable, as well as the most volatile and reactive form.[1]
Citations
- 1 2 Greenwood & Earnshaw 2002, pp. 479, 482
- 1 2 Eagleson 1994, p. 820
- 1 2 Oxtoby, Gillis & Campion 2008, p. 508
- ↑ Sukys 1999, p. 60
- ↑ Bettelheim et al. 2010, p. 37
- ↑ Schulze-Makuch & Irwin 2008, p. 89
- ↑ Steurer 2007, p. 7
- ↑ Brown & Rogers 1987, p. 40
- ↑ Kneen, Rogers & Simpson, 1972, p. 263
- ↑ Stwertka 2012, p. 104
- ↑ Patten 1989, p. 192
- ↑ Russell & Lee 2005, p. 419
- ↑ Cracolice & Peters 2011, p. 335
- ↑ Cracolice & Peters 2011, p. 336
- ↑ Meyer et al. 2005, p. 284; Manahan 2001, p. 911; Szpunar et al. 2004, p. 17
- ↑ Emsley 1971, p. 1
- ↑ Addison 1964, pp. 41–2, 51, 61–3
- ↑ Brady & Senese 2009, pp. 858−63
- ↑ Ball 2013
- ↑ Aylward & Findlay, p. 124
- ↑ Shipman, Wilson & Todd 2009, p. 297
- ↑ Russell & Lee 2005, p. 5; Zumdahl & DeCoste 2013, pp. 35, 784
- ↑ Borg & Dienes 1992, p. 15
- ↑ Wells 1984, pp. 1275, 77
- ↑ Russell & Lee 2005, p. 5
- ↑ Patterson, Kuper & Nanney 1967, p. 388; King 2004, p. 197; DeKock & Gray 1989, p. 426
- ↑ Ashford 1967, p. 329
- ↑ Townes 1952
- ↑ Steudel 1977, p. 240; Greenwood & Earnshaw 2002, p. 803; Wiberg 2001, p. 416
- ↑ Stein 1983, p. 165
- ↑ Wiberg 2001, p. 680
- ↑ Taylor 1960, p. 377; Miller 1987, p. 62; Irving 2005, p. 131
- 1 2 Partington 1944, p. 405
- ↑ Labes et al. 1979
- ↑ Steudel & Eckert 2003, p. 60
- ↑ Mitchell 2006, p. 24
- ↑ Wiberg 2001, p. 517
- ↑ Greenwood & Earnshaw 2002, pp. 664–5
- ↑ Phillips & Williams 1965, pp. 577, 580–1, 583–4
- ↑ Eastman et al. 1950, p. 2250
- ↑ Krikorian & Curtis 1988
- ↑ Cotton 2006, pp. 29–30, 32
- ↑ Martin & Lander 1946, p. 195
- ↑ Dias et al. 2011
- ↑ Winkler 2009, pp. 16, 139
- ↑ Winkler et al. 2011
- ↑ Yu & Cardona 2010, p. 1
- ↑ Moss 1952, pp. 180–84
- ↑ Scharfe & Schmidlin 1975, p. 83
- ↑ Steudel 2003, pp. 106–7
- ↑ Schaefer 1968, p. 76
- ↑ Fujimori et al. 2013
- ↑ Phifer 2000, p. 1
- ↑ Rao 2002, p. 22
- ↑ McMillan 2006
- 1 2 Lide 2003
- ↑ Steudel & Strauss 1984, p. 135
- ↑ Novak 1979, p. 281
- ↑ Chapman & Jarvis 2003, p. 23
- ↑ Wiberg 2001, p. 686
- ↑ Shipman, Wilson & Todd 2009, p. 293
- ↑ Cairns 2012, p. 147
- ↑ Wiberg 2001, p. 416
- ↑ Rogers 2012, p. pp. 267–269; Murray 1976, p. 103–4
- ↑ Greenwood & Earnshaw 2002, pp. 800,804
- ↑ Nelson 1998, p. 25
- ↑ Rayner-Canham & Overton 2006, p. 353
- ↑ Jolly 1966, p. 20
- ↑ Clugston & Flemming 2000, pp. 100–1, 104–5, 302
- ↑ Seaborg 1969, p. 626
- ↑ Nash 2005
- ↑ Scerri 2013, pp. 204–8
- ↑ Kratz, J. V. (5 September 2011). The Impact of Superheavy Elements on the Chemical and Physical Sciences (PDF). 4th International Conference on the Chemistry and Physics of the Transactinide Elements. Retrieved 27 August 2013.
- ↑ Ivanenko et al. 2011, p. 784
- ↑ Catling 2013, p. 12
- 1 2 Crawford 1968, p. 540
- ↑ Merchant & Helmann 2012, pp. 94–96
- ↑ Berkowitz 2012, p. 293
- ↑ Jørgensen & Mitsch 1983, p. 59
- ↑ Wiberg 2001, p. 245–246; Silvera & Walraven 1981, pp. 204–207
- ↑ Wiberg 2001, p. 246
- ↑ Oka 2006; McCall & Oka 2003; Mitchell & McGowan 1983, 310
- ↑ Patil, Dhumal & Gejji 2004
- ↑ Aylward & Findlay, p. 127–129
- ↑ Atkins & Paula p. 355; Raju 2005, p. 495; Bunge & Bunge 1979
- ↑ Bryson 1989, p. 511
- ↑ Desch 1914, p. 86
- ↑ Sherwin & Weston 1966, p. 100, 123, 152; Smith 2011, p. 754; Finney 2015, p. 88
- ↑ Challoner 2014, p. 5; Myers, Oldham & Tocci 2004, pp. 120–121: The latter authors categorize nonmetals as hydrogen; semiconductors “(also known as metalloids)”; other nonmetals (C, N, O, P, S, Se); halogens; or noble gases; Government of Canada 2015; Gargaud et al. 2006, p. 447
- ↑ Jorgensen 2012, p. 56
- ↑ Chung 1987; Godfrin & Lauter 1995
- ↑ Cambridge Enterprise 2013
- ↑ Faraday 1853, p. 42; Holderness & Berry 1979, p. 255
- ↑ Regnault 1853, p. 208
- ↑ Siebring & Schaff 1980, p. 276
- ↑ Conroy 1968, p. 672
- ↑ Jenkins & Kawamura 1976, p. 88
- 1 2 Bogoroditskii & Pasynkov 1967, p. 77
- ↑ Greenwood & Earnshaw 2002, p. 804
- ↑ Stein 1969; Pitzer 1975; Schrobilgen 2011
- ↑ Arunan et al. 2011, p. 1623–4
- ↑ Henderson 2000, p. 134
- ↑ Ritter 2011, p. 10
- ↑ Cacace, de Petris & Troiani 2002
- ↑ Koziel 2002, p. 18
- ↑ Piro et al. 2006
- ↑ Steudel & Eckert 2003, p. 1
- ↑ Greenwood & Earnshaw 2002, pp. 659–660
- ↑ Moss 1952, p. 192; Greenwood & Earnshaw 2002, p. 751
- ↑ Shanabrook, Lannin & Hisatsune 1981
- ↑ Yousuf 1998, p. 425
- ↑ Ostriker & Steinhardt 2001
- ↑ Nelson 1987, p. 732
- ↑ Emsley 2001, p. 428
- ↑ Bolin 2012, p. 2-1
- ↑ Maroni 1995
- ↑ King & Caldwell 1954, p. 17; Brady & Senese 2009, p. 69
- ↑ Nelson 1987, p. 735
References
- Addison WE 1964, The allotropy of the elements, Oldbourne Press, London
- Arunan E, Desiraju GR, Klein RA, Sadlej J, Scheiner S, Alkorta I, Clary DC, Crabtree RH, Dannenberg JJ, Hobza P, Kjaergaard HG, Legon AC, Mennucci B & Nesbitt DJ 2011, "Defining the hydrogen bond: An account (IUPAC Technical Report)", Pure and Applied Chemistry, vol. 83, no. 8, pp. 1619–36, doi:10.1351/PAC-REP-10-01-01
- Ashford TA 1967, The physical sciences: From atoms to stars, 2nd ed., Holt, Rinehart and Winston, New York
- Atkins P & de Paula J 2011, Physical chemistry for the life sciences, 2nd ed., Oxford University Press, Oxford, ISBN 978-1429231145
- Aylward G & Findlay T 2008, SI chemical data, 6th ed., John Wiley & Sons Australia, Milton, Queensland
- Ball P 2013, "The name's bond", Chemistry World, vol. 10, no. 6, p. 41
- Berkowitz J 2012, The stardust revolution: the new story of our origin in the stars, Prometheus Books, Amherst, New York, ISBN 978-1-61614-549-1
- Bettelheim FA, Brown WH, Campbell MK, Farrell SO 2010, Introduction to general, organic, and biochemistry, 9th ed., Brooks/Cole, Belmont California, ISBN 9780495391128
- Bogoroditskii NP & Pasynkov VV 1967, Radio and electronic materials, Iliffe Books, London
- Bolin P 2000, "Gas-insulated substations, in JD McDonald (ed.), Electric power substations engineering, 3rd, ed., CRC Press, Boca Raton, FL, pp. 2–1–2-19, ISBN 9781439856383
- Borg RJ & Dienes GJ 1992, The physical chemistry of solids, Academic Press, San Diego, California, ISBN 9780121184209
- Brady JE & Senese F 2009, Chemistry: The study of matter and its changes, 5th ed., John Wiley & Sons, New York, ISBN 9780470576427
- Brown WH & Rogers EP 1987, General, organic and biochemistry, 3rd ed., Brooks/Cole, Monterey, California, ISBN 0534068707
- Bryson PD 1989, Comprehensive review in toxicology, Aspen Publishers, Rockville, Maryland, ISBN 0871897776
- Bunge AV & Bunge CF 1979, "Electron affinity of helium (1s2s)3S", Physical Review A, vol. 19, no. 2, pp. 452–456, doi:10.1103/PhysRevA.19.452
- Cacace F, de Petris G & Troiani A 2002, "Experimental detection of tetranitrogen", Science, vol. 295, no. 5554, pp. 480–81, doi:10.1126/science.1067681
- Cairns D 2012, Essentials of pharmaceutical chemistry, 4th ed., Pharmaceutical Press, London, ISBN 9780853699798
- Cambridge Enterprise 2013, "Carbon 'candy floss' could help prevent energy blackouts", Cambridge University, viewed 28 August 2013
- Catling DC 2013, Astrobiology: A very short introduction, Oxford University Press, Oxford, ISBN 978-0-19-958645-5
- Challoner J 2014, The elements: The new guide to the building blocks of our universe, Carlton Publishing Group, ISBN 978-0-233-00436-5
- Chapman B & Jarvis A 2003, Organic chemistry, kinetics and equilibrium, rev. ed., Nelson Thornes, Cheltenham, ISBN 978-0-7487-7656-6
- Chung DD 1987, "Review of exfoliated graphite", Journal of Materials Science, vol. 22, pp. 4190–98, doi:10.1007/BF01132008
- Clugston MJ & Flemming R 2000, Advanced chemistry, Oxford University Press, Oxford, ISBN 9780199146338
- Conroy EH 1968, "Sulfur", in CA Hampel (ed.), The encyclopedia of the chemical elements, Reinhold, New York, pp. 665–680
- Cotton S 2006, Lanthanide and actinide chemistry, 2nd ed., John Wiley & Sons, New York, ISBN 9780470010068
- Cracolice MS & Peters EI 2011, Basics of introductory chemistry: An active learning approach, 2nd ed., Brooks/Cole, Belmont California, ISBN 9780495558507
- Crawford FH 1968, Introduction to the science of physics, Harcourt, Brace & World, New York
- DeKock RL & Gray HB 1989, Chemical structure and bonding, 2nd ed., University Science Books, Mill Valley, California, ISBN 093570261X
- Desch CH 1914, Intermetallic Compounds, Longmans, Green and Co., New York
- Dias RP, Yoo C, Kim M & Tse JS 2011, "Insulator-metal transition of highly compressed carbon disulfide," Physical Review B, vol. 84, pp. 144104–1–6, doi:10.1103/PhysRevB.84.144104
- Eagleson M 1994, Concise encyclopedia chemistry, Walter de Gruyter, Berlin, ISBN 3110114518
- Eastman ED, Brewer L, Bromley LA, Gilles PW, Lofgren NL 1950, "Preparation and properties of refractory cerium sulfides", Journal of the American Chemical Society, vol. 72, no. 5, pp. 2248–50, doi:10.1021/ja01161a102
- Emsley J 1971, The inorganic chemistry of the non-metals, Methuen Educational, London, ISBN 0423861204
- Emsley J 2001, Nature's building blocks: An A–Z guide to the elements, Oxford University Press, Oxford, ISBN 0198503415
- Faraday M 1853, The subject matter of a course of six lectures on the non-metallic elements, (arranged by J Scoffern), Longman, Brown, Green, and Longmans, London
- Finney J 2015, Water: A Very Short Introduction, Oxford University Press, Oxford, ISBN 978-0198708728,
- Fujimori T, Morelos-Gómez A, Zhu Z, Muramatsu H, Futamura R, Urita K, Terrones M, Hayashi T, Endo M, Hong SY, Choi YC, Tománek D & Kaneko K 2013, "Conducting linear chains of sulphur inside carbon nanotubes", Nature Communications, vol. 4, article no. 2162, doi:10.1038/ncomms3162
- Gargaud M, Barbier B, Martin H & Reisse J (eds) 2006, Lectures in astrobiology, vol. 1, part 1: The early Earth and other cosmic habitats for life, Springer, Berlin, ISBN 3-540-29005-2
- Government of Canada 2015, Periodic table of the elements, accessed 30 August 2015
- Godfrin H & Lauter HJ 1995, "Experimental properties of 3He adsorbed on graphite", in WP Halperin (ed.), Progress in low temperature physics, volume 14, pp. 213–320 (216–8), Elsevier Science B.V., Amsterdam, ISBN 9780080539935
- Greenwood NN & Earnshaw A 2002, Chemistry of the elements, 2nd ed., Butterworth-Heinemann, ISBN 0750633654
- Henderson W 2000, Main group chemistry, Royal Society of Chemistry, Cambridge, ISBN 9780854046171
- Holderness A & Berry M 1979, Advanced level inorganic chemistry, 3rd ed., Heinemann Educational Books, London, ISBN 9780435654351
- Irving KE 2005, "Using chime simulations to visualize molecules", in RL Bell & J Garofalo (eds), Science units for Grades 9–12, International Society for Technology in Education, Eugene, Oregon, ISBN 9781564842176
- Ivanenko NB, Ganeev AA, Solovyev ND & Moskvin LN 2011, "Determination of trace elements in biological fluids", Journal of Analytical Chemistry, vol. 66, no. 9, pp. 784–799 (784), doi:10.1134/S1061934811090036
- Jenkins GM & Kawamura K 1976, Polymeric carbons—carbon fibre, glass and char, Cambridge University Press, Cambridge, ISBN 0521206936
- Jolly WL 1966, The chemistry of the non-metals, Prentice-Hall, Englewood Cliffs, New Jersey
- Jorgensen CK 2012, Oxidation numbers and oxidation states, Springer-Verlag, Berlin, ISBN 978-3-642-87760-5
- Jørgensen SE & Mitsch WJ (eds) 1983, Application of ecological modelling in environmental management, part A, Elsevier Science Publishing, Amsterdam, ISBN 0-444-42155-6
- King RB 2004, "The metallurgist's periodic table and the Zintl-Klemm concept", in DH Rouvray & BR King (eds), The periodic table: into the 21st century, Research Studies Press, Philadelphia, pp. 189–206, ISBN 0863802923
- King GB & Caldwell WE 1954, The fundamentals of college chemistry, American Book Company, New York
- Kneen WR, Rogers MJW & Simpson P 1972, Chemistry: Facts, patterns, and principles, Addison-Wesley, London, ISBN 0201037793
- Koziel JA 2002, "Sampling and sample preparation for indoor air analysis", in J Pawliszyn (ed.), Comprehensive analytical chemistry, vol. 37, Elsevier Science B.V., Amsterdam, pp. 1–32, ISBN 0444505105
- Krikorian OH & Curtis PG 1988, "Synthesis of CeS and interactions with molten metals", High Temperatures-High Pressures, vol. 20, pp. 9–17, ISSN 0018-1544
- Labes MM, Love P & Nichols LF 1979, "Polysulfur nitride—a metallic, superconducting polymer", Chemical Review, vol. 79, no. 1, pp. 1–15, doi:10.1021/cr60317a002
- Lide DR (ed.) 2003, CRC handbook of chemistry and physics, 84th ed., CRC Press, Boca Raton, Florida, Section 6, Fluid properties; Vapor pressure, ISBN 0849304849
- Manahan SE 2001, Fundamentals of environmental chemistry, 2nd ed., CRC Press, Boca Raton, Florida, ISBN 156670491X
- Maroni M, Seifert B & Lindvall T (eds) 1995, "Physical pollutants", in Indoor air quality: A comprehensive reference book, Elsevier, Amsterdam, pp. 108–123, ISBN 0444816429
- Martin RM & Lander GD 1946, Systematic inorganic chemistry: From the standpoint of the periodic law, 6th ed., Blackie & Son, London
- McCall BJ & Oka T 2003, "Enigma of H3+ in diffuse interstellar clouds", in SL Guberman (ed.), Dissociative recombination of molecular ions with electrons, Springer Science+Business Media, New York, ISBN 978-1-4613-4915-0
- McMillan PF 2006, "Solid-state chemistry: A glass of carbon dioxide", Nature, vol. 441, p. 823, doi:10.1038/441823a
- Merchant SS & Helmann JD 2012, "Elemental economy: Microbial strategies for optimizing growth in the face of nutrient limitation", in Poole RK (ed), Advances in Microbial Physiology, vol. 60, pp. 91–210, doi:10.1016/B978-0-12-398264-3.00002-4
- Meyer JS, Adams WJ, Brix KV, Luoma SM, Mount DR, Stubblefield WA & Wood CM (eds) 2005, Toxicity of dietborne metals to aquatic organisms, Proceedings from the Pellston Workshop on Toxicity of Dietborne Metals to Aquatic Organisms, 27 July–1 August 2002, Fairmont Hot Springs, British Columbia, Canada, Society of Environmental Toxicology and Chemistry, Pensacola, Florida, ISBN 1880611708
- Miller T 1987, Chemistry: a basic introduction, 4th ed., Wadsworth, Belmont, California, ISBN 0534069126
- Mitchell JBA & McGowan JW 1983, "Experimental studies of electron-ion combination", Physics of ion-ion and electron-ion collisions, F Brouillard F & JW McGowan (eds), Plenum Press, ISBN 978-1-4613-3547-4
- Mitchell SC 2006, "Biology of sulfur", in SC Mitchell (ed.), Biological interactions of sulfur compounds, Taylor & Francis, London, pp. 20–41, ISBN 0203375122
- Moss T 1952, Photoconductivity in the elements, Butterworths Scientific Publications, London
- Murray PRS & Dawson PR 1976, Structural and comparative inorganic chemistry: A modern approach for schools and colleges, Heinemann Educational Book, London, ISBN 9780435656447
- Myers RT, Oldham KB & Tocci S 2004, Holt Chemistry, teacher ed., Holt, Rinehart & Winston, Orlando, ISBN 0-03-066463-2
- Nash CS 2005, "Atomic and molecular properties of elements 112, 114, and 118", Journal of Physical Chemistry A, vol. 109, pp. 3493–500, doi:10.1021/jp050736o
- Nelson PG 1987, "Important elements", Journal of Chemical Education, vol. 68, no. 9, pp. 732–737, doi:10.1021/ed068p732
- Nelson PG 1998, "Classifying substances by electrical character: An alternative to classifying by bond type", Journal of Chemical Education, vol. 71, no. 1, pp. 24–6, doi:10.1021/ed071p24
- Novak A 1979, "Vibrational spectroscopy of hydrogen bonded systems", in TM Theophanides (ed.), Infrared and Raman spectroscopy of biological molecules, proceedings of the NATO Advanced Study Institute held at Athens, Greece, August 22–31, 1978, D. Reidel Publishing Company, Dordrecht, Holland, pp. 279–304, ISBN 9027709661
- Oka T 2006, "Interstellar H+
3", PNAS, vol. 103, no. 33, doi:10.1073_pnas.0601242103 - Ostriker JP & Steinhardt PJ 2001, "The quintessential universe", Scientific American, January, pp. 46–53
- Oxtoby DW, Gillis HP & Campion A 2008, Principles of modern chemistry, 6th ed., Thomson Brooks/Cole, Belmont, California, ISBN 0534493661
- Partington JR 1944, A text-book of inorganic chemistry, 5th ed., Macmillan & Co., London
- Patil UN, Dhumal NR & Gejji SP 2004, "Theoretical studies on the molecular electron densities and electrostatic potentials in azacubanes", Theoretica Chimica Acta, vol. 112, no. 1, pp 27–32, doi:10.1007/s00214-004-0551-2
- Patten MN 1989, Other metals and some related materials, in MN Patten (ed.), Information sources in metallic materials, Bowker-Saur, London, ISBN 0408014911
- Patterson CS, Kuper HS & Nanney TR 1967, Principles of chemistry, Appleton Century Crofts, New York
- Phifer C 2000, "Ceramics, glass structure and properties", in Kirk-Othmer Encyclopedia of Chemical Technology, doi:10.1002/0471238961.0712011916080906.a01
- Phillips CSG & Williams RJP 1965, Inorganic chemistry, I: Principles and non-metals, Clarendon Press, Oxford
- Piro NA, Figueroa JS, McKellar JT & Troiani CC 2006, "Triple-bond reactivity of diphosphorus molecules", Science, vol. 313, no. 5791, pp. 1276–9, doi:10.1126/science.1129630
- Pitzer K 1975, "Fluorides of radon and elements 118", Journal of the Chemical Society, Chemical Communications, no. 18, pp. 760–1, doi:10.1039/C3975000760B
- Raju GG 2005, Gaseous Electronics: Theory and Practice, CRC Press, Boca Raton, Florida, ISBN 978-0-203-02526-0
- Rao KY 2002, Structural chemistry of glasses, Elsevier, Oxford, ISBN 0080439586
- Rayner-Canham G & Overton T 2006, Descriptive inorganic chemistry, 4th ed., WH Freeman, New York, ISBN 0716789639
- Regnault MV 1853, Elements of chemistry, vol. 1, 2nd ed., Clark & Hesser, Philadelphia
- Ritter SK 2011, "The case of the missing xenon", Chemical & Engineering News, vol. 89, no. 9, ISSN 0009-2347
- Rodgers GE 2012, Descriptive inorganic, coordination, & solid-state chemistry, 3rd ed., Brooks/Cole, Belmont, California, ISBN 9780840068460
- Russell AM & Lee KL 2005, Structure-property relations in nonferrous metals, Wiley-Interscience, New York, ISBN 047164952X
- Scerri E 2013, A tale of seven elements, Oxford University Press, Oxford, ISBN 9780195391312
- Schaefer JC 1968, "Boron" in CA Hampel (ed.), The encyclopedia of the chemical elements, Reinhold, New York, pp. 73–81
- Scharfe ME & Schmidlin FW 1975, "Charged pigment xerography", in L Marton (ed.), Advances in Electronics and Electron Physics, vol. 38, Academic Press, New York, ISBN 0-12-014538-3, pp. 93–147
- Schrobilgen GJ 2011, "radon (Rn)", in Encyclopædia Britannica, accessed 7 Aug 2011
- Schulze-Makuch D & Irwin LN 2008, Life in the Universe: Expectations and constraints, 2nd ed., Springer-Verlag, Berlin, ISBN 9783540768166
- Seaborg GT 1969, "Prospects for further considerable extension of the periodic table", Journal of Chemical Education, vol. 46, no. 10, pp. 626–34, doi:10.1021/ed046p626
- Shanabrook BV, Lannin JS & Hisatsune IC 1981, "Inelastic light scattering in a onefold-coordinated amorphous semiconductor", Physical Review Letters, vol. 46, no. 2, 12 January, pp. 130–133
- Sherwin E & Weston GJ 1966, Chemistry of the non-metallic elements, Pergamon Press, Oxford
- Shipman JT, Wilson JD & Todd AW 2009, An introduction to physical science, 12th ed., Houghton Mifflin Company, Boston, ISBN 9780618935963
- Siebring BR & Schaff ME 1980, General chemistry, Wadsworth Publishing, Belmont, California
- Silvera I & Walraven JTM 1981, "Monatomic hydrogen – a new stable gas", New Scientist, 22 January
- Smith MB 2011, Organic Chemistry: An Acid—Base Approach, CRC Press, Boca Raton, Florida, ISBN 978-1-4200-7921-0
- Stein L 1969, "Oxidized radon in halogen fluoride solutions", Journal of the American Chemical Society, vol. 19, no. 19, pp. 5396–7, doi:10.1021/ja01047a042
- Stein L 1983, "The chemistry of radon", Radiochimica Acta, vol. 32, pp. 163–71
- Steudel R 1977, Chemistry of the non-metals: With an introduction to atomic structure and chemical bonding, Walter de Gruyter, Berlin, ISBN 3110048825
- Steudel R 2003, "Liquid sulfur", in R Steudel (ed.), Elemental sulfur and sulfur-rich compounds I, Springer-Verlag, Berlin, pp. 81–116, ISBN 9783540401919
- Steudel R & Eckert B 2003, "Solid sulfur allotropes", in R Steudel (ed.), Elemental sulfur and sulfur-rich compounds I, Springer-Verlag, Berlin, pp. 1–80, ISBN 9783540401919
- Steudel R & Strauss E 1984, "Homcyclic selenium molecules and related cations", in HJ Emeleus (ed.), Advances in inorganic chemistry and radiochemistry, vol. 28, Academic Press, Orlando, Florida, pp. 135–167, ISBN 9780080578774
- Steurer W 2007, "Crystal structures of the elements" in JW Marin (ed.), Concise encyclopedia of the structure of materials, Elsevier, Oxford, pp. 127–45, ISBN 0080451276
- Stwertka A 2012, A guide to the elements, 3rd ed., Oxford University Press, Oxford, ISBN 9780199832521
- Sukys P 1999, Lifting the scientific veil: Science appreciation for the nonscientist, Rowman & Littlefield, Oxford, ISBN 0847696006
- Szpunar J, Bouyssiere B & Lobinski R 2004, "Advances in analytical methods for speciation of trace elements in the environment", in AV Hirner & H Emons (eds), Organic metal and metalloid species in the environment: Analysis, distribution processes and toxicological evaluation, Springer-Verlag, Berlin, pp. 17–40, ISBN 3540208291
- Taylor MD 1960, First principles of chemistry, Van Nostrand, Princeton, New Jersey
- Townes CH & Dailey BP 1952, "Nuclear quadrupole effects and electronic structure of molecules in the solid state", Journal of Chemical Physics, vol. 20, pp. 35–40, doi:10.1063/1.1700192
- Wells AF 1984, Structural inorganic chemistry, 5th ed., Clarendon Press, Oxfordshire, ISBN 0198553706
- Wiberg N 2001, Inorganic chemistry, Academic Press, San Diego, ISBN 0123526515
- Winkler MT 2009, "Non-equilibrium chalcogen concentrations in silicon: Physical structure, electronic transport, and photovoltaic potential", PhD thesis, Harvard University, Cambridge, Massachusetts
- Winkler MT, Recht D, Sher M, Said AJ, Mazur E & Aziz MJ 2011, "Insulator-to-metal transition in sulfur-doped silicon", Physical Review Letters, vol. 106, pp. 178701–4
- Yousuf M 1998, "Diamond anvil cells in high-pressure studies of semiconductors", in T Suski & W Paul (eds), High pressure in semiconductor physics II, Semiconductors and semimetals, vol. 55, Academic Press, San Diego, pp. 382–436, ISBN 9780080864532
- Yu PY & Cardona M 2010, Fundamentals of semiconductors: Physics and materials properties, 4th ed., Springer, Heidelberg, ISBN 9783642007101
- Zumdahl SS & DeCoste DJ 2013, Chemical principles, 7th ed., Brooks/Cole, Belmont, California, ISBN 9781111580650
Monographs
- Emsley J 1971, The inorganic chemistry of the non-metals, Methuen Educational, London, ISBN 0423861204
- Johnson RC 1966, Introductory descriptive chemistry: selected nonmetals, their properties, and behavior, WA Benjamin, New York
- Jolly WL 1966, The chemistry of the non-metals, Prentice-Hall, Englewood Cliffs, New Jersey
- Powell P & Timms PL 1974, The chemistry of the non-metals, Chapman & Hall, London, ISBN 0470695706
- Sherwin E & Weston GJ 1966, Chemistry of the non-metallic elements, Pergamon Press, Oxford
- Steudel R 1977, Chemistry of the non-metals: with an introduction to atomic structure and chemical bonding, English edition by FC Nachod & JJ Zuckerman, Berlin, Walter de Gruyter, ISBN 3110048825

