Nociceptin
 | |
| Names | |
|---|---|
| Other names
Orphanin FQ | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| MeSH | nociceptin |
| PubChem CID |
|
| |
| |
| Properties | |
| C79H129N27O22 | |
| Molar mass | 1809.04 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
| prepronociceptin | |
|---|---|
| Identifiers | |
| Symbol | PNOC |
| Entrez | 5368 |
| HUGO | 9163 |
| OMIM | 601459 |
| RefSeq | NM_006228 |
| UniProt | Q13519 |
| Other data | |
| Locus | Chr. 8 p21 |
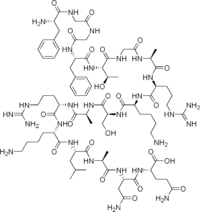
Nociceptin/orphanin FQ (N/OFQ), a 17-amino acid neuropeptide, is the endogenous ligand for the nociceptin receptor (NOP, ORL-1), and initiates its function to act on numerous brain activities such as pain sensation and fear learning. It is derived from the prepronociceptin protein, as are a further 2 peptides, nocistatin & NocII, which inhibit the N/OFQ receptor function.[1] Nociceptin itself acts as a potent anti-analgesic, effectively counteracting the effect of pain-relievers. The gene coding for prepronociceptin is located on Ch8p21 in humans.[2] Nociceptin acts at the Nociceptin receptor (NOP receptor) formerly known as ORL1. Nociceptin is the first example of reverse pharmacology; the NOP receptor was discovered before the endogenous ligand which was discovered by two separate groups in 1995.[3]
Roles of Nociceptin
Since its discovery, nociceptin has been of great interest to researchers. Nociceptin is a peptide related to the opioid class of compounds (ex. morphine and codeine), but it does not act at the classic opioid receptors (namely, mu, kappa, and delta opioid receptors) which typically act as pain relievers. Nociceptin is widely distributed in the CNS; it is found in the hypothalamus, brainstem, and forebrain, as well as in the ventral and dorsal horns of the spinal cord. The NOP receptor is also widely distributed throughout areas of the brain, including the cortex, anterior olfactory nucleus, lateral septum, hypothalamus, hippocampus, amygdala, central gray, pontine nuclei, interpeduncular nucleus, substantia nigra, raphe complex, locus coeruleus, and spinal cord.[4]
Pain
The N/OFQ-NOP system is found in central and peripheral nervous tissue, where it is well placed to modulate nociception, or the body's sensation of pain.[2] Unlike morphine and other opioids that are used to alleviate pain, nociceptin's role in nociception is not straightforward. Administration of N/OFQ in the brain causes increased sensations of pain (hyperalgesia).[3] This makes it unique from classic opioid peptides, which typically act as analgesics (pain relievers), as it means that nociceptin can even counteract analgesia, thus acting as an antiopioid. Additionally, blocking the nociceptin receptor can lead to an increased pain threshold and a decreased tolerance development to analgesic opioids. As such, nociceptin has a lower risk of addiction than many pain relievers that are currently used.[5] Recent studies have proposed that this anti-analgesic function of nociceptin stems from the inhibition of the periaqueductal grey, which controls pain modulation from the central nervous system. This effect of nociceptin may lead to its future use as a method to reduce morphine dosage and decrease the development of tolerance and dependence.[4] When administered to the spinal cord, nociceptin produces similar analgesic effects to classical opioids.[6]
Mood Disorders
There are various studies on animals that suggest that the N/OFQ-NOP system has a part to play in both anxiety and depression.[7] It appears that nociceptin is an anxiolytic (anxiety inhibitor) but also seems to perpetuate depression, since preventing N/OFQ from binding to NOP seems to improve depression.[8][9]
Drug Abuse Medications
The NOP receptor has shown potential as a target for medications designed to alleviate the effects of substance abuse disorders. Areas in the hypothalamus and amygdala that correlate to the reward process of drug abuse have been found to contain NOP receptors. Nociceptin has also been found to inhibit dopamine production related to the reward process. Specifically, nociceptin acts to inhibit neural rewards induced by drugs such as amphetamines, morphine, cocaine, and especially alcohol in animal models, though the exact mechanism of this has not yet been proven. Additionally, nociceptin may have lower tolerance development than drugs such as morphine. This was shown when nociceptin compounds were used as a pain medication substitution for morphine. Nociceptin also has therapeutic capabilities for addictions to multiple drugs, potentially playing a role in compounds that have decreased withdrawal tendencies (such as muscle aches, anxiety, and restlessness).[5]
Learning and Memory
In animal studies, the N/OFQ-NOP receptor pathway has also been found to play both positive and negative roles in both learning and memory. For example, malfunctions in this pathway are linked to altered fear learning in brain disorders such as post-traumatic stress disorder (PTSD). As such, the receptor pathway maintains homeostatic responses to fear and stressful situations.[10] Nociceptin could also play an inhibitory role in memory function, as some studies show that it impairs spatial learning in vivo, while inhibiting long term potentiation and synaptic transmission in vitro.[4]
Cardiovascular System
The N/OFQ-NOP system has also been implicated in control of the cardiovascular system, as nociceptin administration has led to high blood pressure and bradycardia. Nociceptin has significant effects on cardiovascular parameters such as blood pressure and heart rate that vary by species, as it is excitatory for rodents yet inhibitory for sheep.[4]
Renal System
In the renal system, nociceptin plays a role in water balance, electrolyte balance, and arterial blood pressure regulation. It has also shown potential as a diuretic treatment for alleviating water-retaining diseases.[4]
Immune System
Additional research suggests that nociceptin may be involved in the immune system and sepsis.[11] A study at the University of Leicester looked at patients who were critically ill with sepsis and found that blood N/OFQ levels were significantly higher in patients who died within thirty days in comparison to survivors.[12]
Digestive System
In the gut, nociceptin has been found to have varying effects on stomach and intestinal contractility while also stimulating the increased consumption of food. Additional studies have shown that nociceptin may have an effect as an anti-epileptic drug component.[4]
References
- ↑ Okuda-Ashitaka E, Minami T, Tachibana S, Yoshihara Y, Nishiuchi Y, Kimura T, Ito S (March 1998). "Nocistatin, a peptide that blocks nociceptin action in pain transmission". Nature. 392 (6673): 286–9. PMID 9521323. doi:10.1038/32660.
- 1 2 Mollereau C, Simons MJ, Soularue P, Liners F, Vassart G, Meunier JC, Parmentier M (August 1996). "Structure, tissue distribution, and chromosomal localization of the prepronociceptin gene". Proceedings of the National Academy of Sciences of the United States of America. 93 (16): 8666–70. PMC 38730
 . PMID 8710928. doi:10.1073/pnas.93.16.8666.
. PMID 8710928. doi:10.1073/pnas.93.16.8666. - 1 2 Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B (October 1995). "Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor". Nature. 377 (6549): 532–5. PMID 7566152. doi:10.1038/377532a0.
- 1 2 3 4 5 6 Calo' G, Guerrini R, Rizzi A, Salvadori S, Regoli D (April 2000). "Pharmacology of nociceptin and its receptor: a novel therapeutic target". British Journal of Pharmacology. 129 (7): 1261–83. PMC 1571975
 . PMID 10742280. doi:10.1038/sj.bjp.0703219.
. PMID 10742280. doi:10.1038/sj.bjp.0703219. - 1 2 Zaveri NT (2011-01-01). "The nociceptin/orphanin FQ receptor (NOP) as a target for drug abuse medications". Current Topics in Medicinal Chemistry. 11 (9): 1151–6. PMC 3899399
 . PMID 21050175. doi:10.2174/156802611795371341.
. PMID 21050175. doi:10.2174/156802611795371341. - ↑ Katsuyama S, Mizoguchi H, Komatsu T, Sakurada C, Tsuzuki M, Sakurada S, Sakurada T (July 2011). "Antinociceptive effects of spinally administered nociceptin/orphanin FQ and its N-terminal fragments on capsaicin-induced nociception". Peptides. 32 (7): 1530–5. PMID 21672568. doi:10.1016/j.peptides.2011.05.028.
- ↑ Lambert DG (August 2008). "The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential". Nature Reviews. Drug Discovery. 7 (8): 694–710. PMID 18670432. doi:10.1038/nrd2572.
- ↑ Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Nothacker HP, Civelli O (December 1997). "Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress". Proceedings of the National Academy of Sciences of the United States of America. 94 (26): 14854–8. PMC 25127
 . PMID 9405703. doi:10.1073/pnas.94.26.14854.
. PMID 9405703. doi:10.1073/pnas.94.26.14854. - ↑ Gavioli EC, Vaughan CW, Marzola G, Guerrini R, Mitchell VA, Zucchini S, De Lima TC, Rae GA, Salvadori S, Regoli D, Calo' G (June 2004). "Antidepressant-like effects of the nociceptin/orphanin FQ receptor antagonist UFP-101: new evidence from rats and mice". Naunyn-Schmiedeberg's Archives of Pharmacology. 369 (6): 547–53. PMID 15197534. doi:10.1007/s00210-004-0939-0.
- ↑ Andero R (October 2015). "Nociceptin and the nociceptin receptor in learning and memory". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 62: 45–50. PMC 4458422
 . PMID 25724763. doi:10.1016/j.pnpbp.2015.02.007.
. PMID 25724763. doi:10.1016/j.pnpbp.2015.02.007. - ↑ Thomas R, Stover C, Lambert DG, Thompson JP (October 2014). "Nociceptin system as a target in sepsis?". Journal of Anesthesia. 28 (5): 759–67. PMID 24728719. doi:10.1007/s00540-014-1818-6.
- ↑ Williams JP, Thompson JP, Young SP, Gold SJ, McDonald J, Rowbotham DJ, Lambert DG (June 2008). "Nociceptin and urotensin-II concentrations in critically ill patients with sepsis". British Journal of Anaesthesia. 100 (6): 810–4. PMID 18430746. doi:10.1093/bja/aen093.
External links
- nociceptin at the US National Library of Medicine Medical Subject Headings (MeSH)
- prepronociceptin at the US National Library of Medicine Medical Subject Headings (MeSH)