Nitrogen cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into various chemical forms as it circulates among the atmosphere, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is nitrogen,[1] making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems. The nitrogen cycle is of particular interest to ecologists because nitrogen availability can affect the rate of key ecosystem processes, including primary production and decomposition. Human activities such as fossil fuel combustion, use of artificial nitrogen fertilizers, and release of nitrogen in wastewater have dramatically altered the global nitrogen cycle.[2]

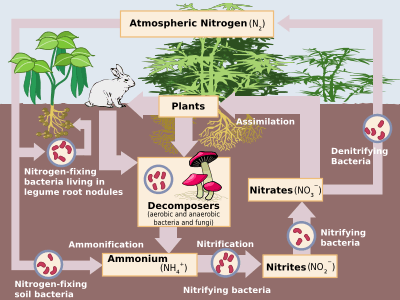
The processes of the nitrogen cycle
Nitrogen is present in the environment in a wide variety of chemical forms including organic nitrogen, Ammonium (NH+
4), nitrite (NO−
2), nitrate (NO−
3), nitrous oxide (N2O), Nitric oxide (NO) or inorganic nitrogen gas (N2). Organic nitrogen may be in the form of a living organism, humus or in the intermediate products of organic matter decomposition. The processes of the nitrogen cycle transform nitrogen from one form to another. Many of those processes are carried out by microbes, either in their effort to harvest energy or to accumulate nitrogen in a form needed for their growth. For example, the nitrogenous wastes in animal urine are broken down by nitrifying bacteria in the soil to be used as new. The diagram besides shows how these processes fit together to form the nitrogen cycle.
Nitrogen fixation
Atmospheric nitrogen must be processed, or "fixed", in a usable form to be taken up by plants. Between 5x1012 and 10x1012 g per year are fixed by lightning strikes, but most fixation is done by free-living or symbiotic bacteria known as diazotrophs. These bacteria have the nitrogenase enzyme that combines gaseous nitrogen with hydrogen to produce ammonia, which is converted by the bacteria into other organic compounds. Most biological nitrogen fixation occurs by the activity of Mo-nitrogenase, found in a wide variety of bacteria and some Archaea. Mo-nitrogenase is a complex two-component enzyme that has multiple metal-containing prosthetic groups.[3] An example of the free-living bacteria is Azotobacter. Symbiotic nitrogen-fixing bacteria such as Rhizobium usually live in the root nodules of legumes (such as peas, alfalfa, and locust trees). Here they form a mutualistic relationship with the plant, producing ammonia in exchange for carbohydrates. Because of this relationship, legumes will often increase the nitrogen content of nitrogen-poor soils. A few non-legumes can also form such symbioses. Today, about 30% of the total fixed nitrogen is produced industrially using the Haber-Bosch process,[4] which uses high temperatures and pressures to convert nitrogen gas and a hydrogen source (natural gas or petroleum) into ammonia.[5]
Assimilation
Plants take nitrogen from the soil by absorption through their roots as amino acids, nitrate ions, nitrite ions, or ammonium ions. Most nitrogen obtained by terrestrial animals can be traced back to the eating of plants at some stage of the food chain.
Plants can absorb nitrate or ammonium from the soil via their root hairs. If nitrate is absorbed, it is first reduced to nitrite ions and then ammonium ions for incorporation into amino acids, nucleic acids, and chlorophyll.[5] In plants that have a symbiotic relationship with rhizobia, some nitrogen is assimilated in the form of ammonium ions directly from the nodules. It is now known that there is a more complex cycling of amino acids between Rhizobia bacteroids and plants. The plant provides amino acids to the bacteroids so ammonia assimilation is not required and the bacteroids pass amino acids (with the newly fixed nitrogen) back to the plant, thus forming an interdependent relationship.[6] While many animals, fungi, and other heterotrophic organisms obtain nitrogen by ingestion of amino acids, nucleotides, and other small organic molecules, other heterotrophs (including many bacteria) are able to utilize inorganic compounds, such as ammonium as sole N sources. Utilization of various N sources is carefully regulated in all organisms.
Ammonification
When a plant or animal dies or an animal expels waste, the initial form of nitrogen is organic. Bacteria or fungi convert the organic nitrogen within the remains back into ammonium (NH+
4), a process called ammonification or mineralization. Enzymes involved are:
- GS: Gln Synthetase (Cytosolic & Plastic)
- GOGAT: Glu 2-oxoglutarate aminotransferase (Ferredoxin & NADH-dependent)
- GDH: Glu Dehydrogenase:
- Minor Role in ammonium assimilation.
- Important in amino acid catabolism.
Nitrification
The conversion of ammonium to nitrate is performed primarily by soil-living bacteria and other nitrifying bacteria. In the primary stage of nitrification, the oxidation of ammonium (NH+
4) is performed by bacteria such as the Nitrosomonas species, which converts ammonia to nitrites (NO−
2). Other bacterial species such as Nitrobacter, are responsible for the oxidation of the nitrites (NO−
2) into nitrates (NO−
3).[5] It is important for the ammonia (NH
3) to be converted to nitrates or nitrites because ammonia gas is toxic to plants.
Due to their very high solubility and because soils are highly unable to retain anions, nitrates can enter groundwater. Elevated nitrate in groundwater is a concern for drinking water use because nitrate can interfere with blood-oxygen levels in infants and cause methemoglobinemia or blue-baby syndrome.[7][8] Where groundwater recharges stream flow, nitrate-enriched groundwater can contribute to eutrophication, a process that leads to high algal population and growth, especially blue-green algal populations. While not directly toxic to fish life, like ammonia, nitrate can have indirect effects on fish if it contributes to this eutrophication. Nitrogen has contributed to severe eutrophication problems in some water bodies. Since 2006, the application of nitrogen fertilizer has been increasingly controlled in Britain and the United States. This is occurring along the same lines as control of phosphorus fertilizer, restriction of which is normally considered essential to the recovery of eutrophied waterbodies.
Denitrification
Denitrification is the reduction of nitrates back into nitrogen gas (N2), completing the nitrogen cycle. This process is performed by bacterial species such as Pseudomonas and Clostridium in anaerobic conditions.[5] They use the nitrate as an electron acceptor in the place of oxygen during respiration. These facultatively anaerobic bacteria can also live in aerobic conditions. Denitrification happens in anaerobic conditions e.g. waterlogged soils. The denitrifying bacteria use nitrates in the soil to carry out respiration and consequently produce nitrogen gas, which is inert and unavailable to plants.
Dissimilatory nitrate reduction to ammonium
Dissimilatory nitrate reduction to ammonium (DNRA), or nitrate/nitrite ammonification, is an anaerobic respiration process. Microbes which undertake DNRA oxidise organic matter and use nitrate as an electron acceptor, reducing it to nitrite, then ammonium (NO3−→NO2−→NH4+).[9] Both denitrifying and nitrate ammonification bacteria will be competing for nitrate in the environment, although DNRA acts to conserve bioavailable nitrogen as soluble ammonium rather than producing dinitrogen gas.[10]
Anaerobic ammonia oxidation
In this biological process, nitrite and ammonia are converted directly into molecular nitrogen (N2) gas. This process makes up a major proportion of nitrogen conversion in the oceans. The balanced formula for this "anammox" chemical reaction is: NH+
4 + NO−
2 => N2 + 2H2O (ΔG° = -357 kJ mol-1).[11]
Other processes
Though nitrogen fixation is the primary source of plant-available nitrogen in most ecosystems, in areas with nitrogen-rich bedrock, the breakdown of this rock also serves as a nitrogen source.[12][13][14]
Marine nitrogen cycle
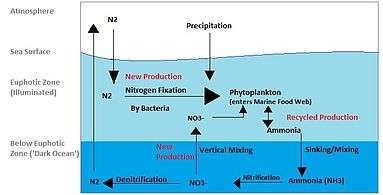
The nitrogen cycle is an important process in the ocean as well. While the overall cycle is similar, there are different players and modes of transfer for nitrogen in the ocean. Nitrogen enters the water through the precipitation, runoff, or as N2 from the atmosphere. Nitrogen cannot be utilized by phytoplankton as N2 so it must undergo nitrogen fixation which is performed predominately by cyanobacteria.[15] Without supplies of fixed nitrogen entering the marine cycle, the fixed nitrogen would be used up in about 2000 years.[16] Phytoplankton need nitrogen in biologically available forms for the initial synthesis of organic matter. Ammonia and urea are released into the water by excretion from plankton. Nitrogen sources are removed from the euphotic zone by the downward movement of the organic matter. This can occur from sinking of phytoplankton, vertical mixing, or sinking of waste of vertical migrators. The sinking results in ammonia being introduced at lower depths below the euphotic zone. Bacteria are able to convert ammonia to nitrite and nitrate but they are inhibited by light so this must occur below the euphotic zone.[17] Ammonification or Mineralization is performed by bacteria to convert organic nitrogen to ammonia. Nitrification can then occur to convert the ammonium to nitrite and nitrate.[18] Nitrate can be returned to the euphotic zone by vertical mixing and upwelling where it can be taken up by phytoplankton to continue the cycle. N2 can be returned to the atmosphere through denitrification.
Ammonium is thought to be the preferred source of fixed nitrogen for phytoplankton because its assimilation does not involve a redox reaction and therefore requires little energy. Nitrate requires a redox reaction for assimilation but is more abundant so most phytoplankton have adapted to have the enzymes necessary to undertake this reduction (nitrate reductase). There are a few notable and well-known exceptions that include Prochlorococcus and some Synechococcus.[16] These species can only take up nitrogen as ammonium.
The nutrients in the ocean are not uniformly distributed. Areas of upwelling provide supplies of nitrogen from below the euphotic zone. Coastal zones provide nitrogen from runoff and upwelling occurs readily along the coast. However, the rate at which nitrogen can be taken up by phytoplankton is decreased in oligotrophic waters year-round and temperate water in the summer resulting in lower primary production.[19] The distribution of the different forms of nitrogen varies throughout the oceans as well.
Nitrate is depleted in near-surface water except in upwelling regions. Coastal upwelling regions usually have high nitrate and chlorophyll levels as a result of the increased production. However, there are regions of high surface nitrate but low chlorophyll that are referred to as HNLC (high nitrogen, low chlorophyll) regions. The best explanation for HNLC regions relates to iron scarcity in the ocean, which may play an important part in ocean dynamics and nutrient cycles. The input of iron varies by region and is delivered to the ocean by dust (from dust storms) and leached out of rocks. Iron is under consideration as the true limiting element to ecosystem productivity in the ocean.
Ammonium and nitrite show a maximum concentration at 50–80 m (lower end of the euphotic zone) with decreasing concentration below that depth. This distribution can be accounted for by the fact that nitrite and ammonium are intermediate species. They are both rapidly produced and consumed through the water column.[16] The amount of ammonium in the ocean is about 3 orders of magnitude less than nitrate.[16] Between ammonium, nitrite, and nitrate, nitrite has the fastest turnover rate. It can be produced during nitrate assimilation, nitrification, and denitrification; however, it is immediately consumed again.
New vs. regenerated nitrogen
Nitrogen entering the euphotic zone is referred to as new nitrogen because it is newly arrived from outside the productive layer.
[15] The new nitrogen can come from below the euphotic zone or from outside sources. Outside sources are upwelling from deep water and nitrogen fixation. If the organic matter is eaten, respired, delivered to the water as ammonia, and re-incorporated into organic matter by phytoplankton it is considered recycled/regenerated production.
New production is an important component of the marine environment. One reason is that only continual input of new nitrogen can determine the total capacity of the ocean to produce a sustainable fish harvest.[20] Harvesting fish from regenerated nitrogen areas will lead to a decrease in nitrogen and therefore a decrease in primary production. This will have a negative effect on the system. However, if fish are harvested from areas of new nitrogen the nitrogen will be replenished.
Human influences on the nitrogen cycle
As a result of extensive cultivation of legumes (particularly soy, alfalfa, and clover), growing use of the Haber–Bosch process in the creation of chemical fertilizers, and pollution emitted by vehicles and industrial plants, human beings have more than doubled the annual transfer of nitrogen into biologically available forms.[8] In addition, humans have significantly contributed to the transfer of nitrogen trace gases from Earth to the atmosphere and from the land to aquatic systems. Human alterations to the global nitrogen cycle are most intense in developed countries and in Asia, where vehicle emissions and industrial agriculture are highest.[21]
Nitrous oxide (N2O) has risen in the atmosphere as a result of agricultural fertilization, biomass burning, cattle and feedlots, and industrial sources.[22] N2O has deleterious effects in the stratosphere, where it breaks down and acts as a catalyst in the destruction of atmospheric ozone. Nitrous oxide is also a greenhouse gas and is currently the third largest contributor to global warming, after carbon dioxide and methane. While not as abundant in the atmosphere as carbon dioxide, it is, for an equivalent mass, nearly 300 times more potent in its ability to warm the planet.[23]
Ammonia (NH3) in the atmosphere has tripled as the result of human activities. It is a reactant in the atmosphere, where it acts as an aerosol, decreasing air quality and clinging to water droplets, eventually resulting in nitric acid (HNO3) that produces acid rain. Atmospheric ammonia and nitric acid also damage respiratory systems.
The very-high temperature of lightning naturally produces small amounts of NOx, NH3, and HNO3, but high-temperature combustion has contributed to a 6 or 7 fold increase in the flux of NOx to the atmosphere. Its production is a function of combustion temperature - the higher the temperature, the more NOx is produced. Fossil fuel combustion is a primary contributor, but so are biofuels and even the burning of hydrogen. The higher combustion temperature of hydrogen produces more NOx than natural gas combustion.
Ammonia and nitrous oxides actively alter atmospheric chemistry. They are precursors of tropospheric (lower atmosphere) ozone production, which contributes to smog and acid rain, damages plants and increases nitrogen inputs to ecosystems.[24] Ecosystem processes can increase with nitrogen fertilization, but anthropogenic input can also result in nitrogen saturation, which weakens productivity and can damage the health of plants, animals, fish, and humans.[8]
Decreases in biodiversity can also result if higher nitrogen availability increases nitrogen-demanding grasses, causing a degradation of nitrogen-poor, species diverse heathlands.[25]
Environmental impacts
Additional risks posed by increased availability of inorganic nitrogen in aquatic ecosystems include water acidification; eutrophication of fresh and saltwater systems; and toxicity issues for animals, including humans.[26] Eutrophication[27] often leads to lower dissolved oxygen levels in the water column, including hypoxic and anoxic conditions, which can cause death of aquatic fauna. Relatively sessile benthos, or bottom-dwelling creatures, are particularly vulnerable because of their lack of mobility, though large fish kills are not uncommon. Oceanic dead zones near the mouth of the Mississippi in the Gulf of Mexico are a well-known example of algal bloom-induced hypoxia.[28][29] The New York Adirondack Lakes, Catskills, Hudson Highlands, Rensselaer Plateau and parts of Long Island display the impact of nitric acid rain deposition, resulting in the killing of fish and many other aquatic species.[30]
Ammonia (NH3) is highly toxic to fish and the level of ammonia discharged from wastewater treatment facilities must be closely monitored. To prevent fish deaths, nitrification via aeration prior to discharge is often desirable. Land application can be an attractive alternative to the aeration.
See also
References
- ↑ Steven B. Carroll; Steven D. Salt (2004). Ecology for gardeners. Timber Press. p. 93. ISBN 978-0-88192-611-8.
- ↑ Galloway, J. N.; et al. (2004). "Nitrogen cycles: past, present, and future generations". Biogeochemistry. 70: 153–226. doi:10.1007/s10533-004-0370-0.
- ↑ Moir, JWB (editor) (2011). Nitrogen Cycling in Bacteria: Molecular Analysis. Caister Academic Press. ISBN 978-1-904455-86-8.
- ↑ Smith, B., R. L. Richards, and W. E. Newton. 2004. Catalysts for nitrogen fixation : nitrogenases, relevant chemical models and commercial processes. Kluwer Academic Publishers, Dordrecht ; Boston.
- 1 2 3 4 Smil, V (2000). Cycles of Life. Scientific American Library, New York.
- ↑ Willey, Joanne M. (2011). Prescott's Microbiology 8th Ed. New York, N.Y.: McGraw Hill. p. 705. ISBN 978-0-07-337526-7.
- ↑ "Nitrogen Cycle".
- 1 2 3 Vitousek, PM; Aber, J; Howarth, RW; Likens, GE; Matson, PA; Schindler, DW; Schlesinger, WH; Tilman, GD (1997). "Human alteration of the global nitrogen cycle: Sources and consequences". Issues in Ecology. 1 (3): 1–17. doi:10.1890/1051-0761(1997)007[0737:HAOTGN]2.0.CO;2.
- ↑ Lam, Phyllis and Kuypers, Marcel M. M. (2011). "Microbial Nitrogen Processes in Oxygen Minimum Zones". Annual Review of Marine Science. 3: 317–345.
- ↑ Marchant, H. K., Lavik, G., Holtappels, M., and Kuypers, M. M. M. (2014). "The Fate of Nitrate in Intertidal Permeable Sediments". PLoS ONE. 9 (8). doi:10.1371/journal.pone.0104517.
- ↑ "Anammox". Anammox - MicrobeWiki. MicrobeWiki. Retrieved 5 July 2015.
- ↑ "Nitrogen Study Could 'Rock' A Plant's World". 2011-09-06. Retrieved 2011-10-22.
- ↑ Schuur, E. A. G. (2011). "Ecology: Nitrogen from the deep". Nature. 477 (7362): 39–40. PMID 21886152. doi:10.1038/477039a.
- ↑ Morford, S. L.; Houlton, B. Z.; Dahlgren, R. A. (2011). "Increased forest ecosystem carbon and nitrogen storage from nitrogen rich bedrock". Nature. 477 (7362): 78–81. PMID 21886160. doi:10.1038/nature10415.
- 1 2 Miller, Charles (2008). Biological Oceanography. 350 Main Street, Malden, MA 02148 USA: Blackwell Publishing Ltd. pp. 60–62. ISBN 978-0-632-05536-4.
- 1 2 3 4 Gruber, Nicolas (2008). Nitrogen in the Marine Environment. 30 Corporate Drive, Suite 400, Burlington, MA 01803: Elsevier. pp. 1–35. ISBN 978-0-12-372522-6.
- ↑ Miller, Charles (2008). Biological oceanography. 350 Main Street, Malden, MA 02148 USA: Blackwell Publishing Ltd. pp. 60–62. ISBN 978-0-632-05536-4.
- ↑ Boyes, Elliot, Susan, Michael. "Learning Unit: Nitrogen Cycle Marine Environment". Retrieved 22 October 2011.
- ↑ Lalli, Parsons, Carol, Timothy (1997). Biological oceanography: An introduction. Butterworth-Heinemann. ISBN 978-0-7506-3384-0.
- ↑ Lalli, Parsons, Carol, Timothy (1997). Biological Oceanography: An introduction. Butterworth-Heinemann. ISBN 978-0-7506-3384-0.
- ↑ Holland, Elisabeth A.; Dentener, Frank J.; Braswell, Bobby H.; Sulzman, James M. (1999). "Contemporary and pre-industrial global reactive nitrogen budgets". Biogeochemistry. 46: 7. doi:10.1007/BF01007572.
- ↑ Chapin, S. F. III, Matson, P. A., Mooney H. A. 2002. Principles of Terrestrial Ecosystem Ecology. Springer, New York 2002 ISBN 0-387-95443-0, p.345
- ↑ Proceedings of the Scientific Committee on Problems of the Environment (SCOPE) International Biofuels Project Rapid Assessment, 22–25 September 2008, Gummersbach, Germany, R. W. Howarth and S. Bringezu, editors. 2009 Executive Summary, p. 3
- ↑ Smil, V (2004). Cycles Of Life: Civilization And The Biosphere. Diane Pub Co. ISBN 0-7567-7350-4.
- ↑ Aerts, Rien & Berendse, Frank (1988). "The Effect of Increased Nutrient Availability on Vegetation Dynamics in Wet Heathlands". Vegetatio. 76 (1/2): 63–69. JSTOR 20038308.
- ↑ Camargo, J.A. & Alonso, A. (2006). Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Retrieved December 10, 2010, from http://www.aseanenvironment.info/Abstract/41013039.pdf
- ↑ "Eutrophication". Wikipedia. 2017-04-14.
- ↑ Rabalais, Nancy N., R. Eugene Turner, and William J. Wiseman, Jr. (2002). "Gulf of Mexico Hypoxia, aka "The Dead Zone"". Annu. Rev. Ecol. Syst. 33: 235–63. JSTOR 3069262. doi:10.1146/annurev.ecolsys.33.010802.150513.
- ↑ Dybas, Cheryl Lyn. (2005). "Dead Zones Spreading in World Oceans". BioScience. 55 (7): 552–557. doi:10.1641/0006-3568(2005)055[0552:DZSIWO]2.0.CO;2.
- ↑ New York State Environmental Conservation, Environmental Impacts of Acid Deposition: Lakes