Nitramide
 | |||
| |||
| Names | |||
|---|---|---|---|
| Other names
Nitramine | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| PubChem CID |
|||
| |||
| |||
| Properties | |||
| H2N2O2 | |||
| Molar mass | 62.03 g mol−1 | ||
| Appearance | colourless solid[1] | ||
| Melting point | 72 to 75 °C (162 to 167 °F; 345 to 348 K)[1] | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
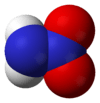
Nitramide, H2NNO2, is a chemical compound. Organyl derivatives of nitramide, RNHNO2 are termed nitroamines, and are widely used as explosives: examples include RDX and HMX.
Structure
The nitramide molecule is essentially an amine group (-NH2) bonded to a nitro group (-NO2). It is reported to be non-planar in the gas phase,[2] but planar in the crystal phase.[1]
Synthesis
Thiele and Lachman's original synthesis of nitramide involved the hydrolysis of potassium nitrocarbamate:[1]
- K2(O2NNCO2) + 2H2SO4 → O2NNH2 + CO2 + 2KHSO4
Other routes to nitramide include hydrolysis of nitrocarbamic acid,
- O2NNHCO2H → O2NNH2 + CO2
reaction of sodium sulfamate with nitric acid,
- Na(SO3NH2) + HNO3 → O2NNH2 + NaHSO4
and reaction of dinitrogen pentoxide with two equivalents of ammonia.
- N2O5 + 2NH3 → O2NNH2 + NH4NO3
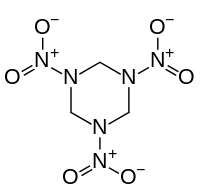
Organic nitramides
Also called nitramines, organic nitramides are important explosives. They are prepared by nitrolysis of hexamethylenetetramine.
References
- 1 2 3 4 Häußler, A.; Klapötke, T. M.; Piotrowski, H. (2002). "Experimental and Theoretical Study on the Structure of Nitramide H2NNO2" (PDF). Zeitschrift für Naturforschung. 57 b (2): 151–156.
- ↑ Tyler, J. K. (1963). "Microwave Spectrum of Nitramide". Journal of Molecular Spectroscopy. 11 (1–6): 39–46. doi:10.1016/0022-2852(63)90004-3.