Neuroscience of sex differences
 |
| This article is one of a series on: |
| Sex differences in humans |
|---|
| Physiology |
| Medicine |
| Sexual differentiation |
| Autism |
| Depression |
| Schizophrenia |
| Stroke care |
| Neuroscience |
| Memory |
| Eyewitness memory |
| Cognition |
| Intelligence |
| Emotional intelligence |
| Psychology |
| Gender psychology |
| Sociology |
| Crime |
| Education |
| Leadership |
| Social capital |
| Suicide |
Neuroscience of sex differences is the study of the characteristics of the brain that separate the male brain and the female brain. Psychological sex differences are thought by some to reflect the interaction of genes, hormones and social learning on brain development throughout the lifespan.
Some evidence from brain morphology and function studies indicates that male and female brains cannot always be assumed to be identical from either a structural or functional perspective, and some brain structures are sexually dimorphic.[1][2]
Experts note that neural sexual dimorphisms in humans exist only as averages, with overlapping variabilities,[3] and that it is unknown to what extent each is influenced by genetics or environment, even in adulthood.[4][5]
History
Ideas of differences in the male and female brain circulated during the time of ancient Greek philosophers around 850 B.C. Aristotle claimed that males did not "receive their soul" until 40 days post-gestation and females did not until 80 days. In 1854, Emil Huschke discovered that "the frontal lobe in the male is all of 1% larger than that of the female."[6] As the 19th century progressed, scientists began researching sexual dimorphisms in the brain significantly more.[7] Until around 21 years ago, scientists knew of several structural sexual dimorphisms of the brain, but they did not think that gender had any impact on how the human brain performs daily tasks. Through molecular, animal, and neuroimaging studies, a great deal of information regarding the differences between male and female brains and how much they differ in regards to both structure and function has been uncovered.[8]
Evolutionary explanations
Sexual selection
It is thought that male and female differences in learning ability have contributed to sexual selection and mate preference throughout evolution. The hippocampus has even been found to exhibit seasonal activity in some mammals where it is active during breeding periods but inactive during hibernation; this is because spatial learning is more present during the breeding season.[9]
Females show enhanced information recall compared to males. This may be due to the fact that females have a more intricate evaluation of risk-scenario contemplation, based on a prefrontal cortical control of the amygdala. For example, the ability to recall information better than males most likely originated from sexual selective pressures on females during competition with other females in mate selection. Recognition of social cues was an advantageous characteristic because it ultimately maximized offspring and was therefore selected for during evolution.[1]
Oxytocin is a hormone that induces contraction of the uterus and lactation in mammals. It is also a characteristic hormone of nursing mothers. Studies have found that oxytocin improves spatial memory. Through activation of the MAP kinase pathway, oxytocin plays a role in the enhancement of long-term synaptic plasticity, which is a change in strength between two neurons over a synapse that lasts for minutes or longer, and long-term memory. This hormone may have helped mothers remember the location of distant food sources so they could better nurture their offspring.[1]
Male vs. female brain anatomy
Hemisphere differences
Inter- and intrahemispheric connectivities are different between male and female.
A popular theory regarding language functions suggests that women use both hemispheres more equally, whereas men are more strongly lateralized to the left hemisphere.[10] This theory found initial support in a high-profile study of 19 men and 19 women, which found stronger lateralization in men during one of the three language tasks assessed.[11] In 2008, some researchers concluded that further studies have failed to replicate this finding, and a meta-analysis of 29 studies comparing language lateralization in males and females found no overall difference.[12] However, in 2013, researchers at the Perelman School of Medicine at the University of Pennsylvania mapped notable differences in male and female neural wiring. The study used diffusion tensor imaging of 949 individuals aged 8–22 years, and concluded that in all supratentorial regions of the brain inter-hemispheric connectivity was greater in women's and girls' brains, whereas intra-hemispheric connectivity was greater in the brains of men and boys. The effect was reversed in cerebellar connections.[13] The detected differences in neural connectivity were negligible up to the age of 13, but became much more prominent in the 14 to 17-year-olds.[13] In terms of the potential effect on behaviour, the authors concluded, "Overall, the results suggest that male brains are structured to facilitate connectivity between perception and coordinated action, whereas female brains are designed to facilitate communication between analytical and intuitive processing modes".[13]
Amygdala
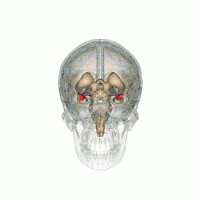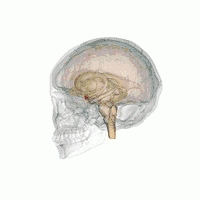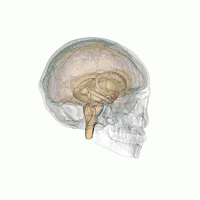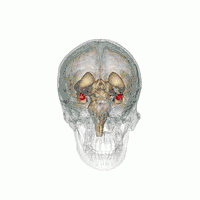
According to some researchers, "the research on sex differences in the amygdala has produced conflicting results".[14] After correcting for the overall difference in brain volume between men and women, a 2016 meta-analysis indicated that the amygdala is not significantly larger in either sex.[15] Some studies, however, report increased amygdala activity during the processing of affective scenes in men relative to women (Schienle et al., 2005; Goldstein et al., 2010), and meta-analysis supports this view, showing larger effect sizes in studies of affective processing including only men compared with those including only women (Sergerie et al., 2008). However several studies using similar stimuli have reported a larger amygdala response in women (Klein et al., 2003; McClure et al., 2004; Hofer et al., 2006; Domes et al., 2010), and others have reported no sex difference at all (Wrase et al., 2003; Caseras et al., 2007; Aleman and Swart, 2008). A possible explanation for these inconsistent results is that sex differences in amygdala response are valence-dependent. Furthermore, according to other researchers,[16] "Correlation analyses revealed that gray matter thickness in left ventromedial PFC was inversely correlated with task-related activation in the amygdala. These data add support to a general role of the ventromedial PFC in regulating activity of the amygdala."
Research has been done on post-traumatic stress disorder (PTSD), an anxiety disorder found in both sexes, which is particularly common in war veterans, assault victims and women who have experienced abuse. Emotional memory encoding varies in the amygdala on the right and left and occurs equally for both genders: the right triggers unpleasant and fear-related memories, both declarative (conscious) and episodic (nonconcious).[17]
Amygdala volume correlates positively with fearfulness in girls but not in boys.[18]
Hippocampus
Several studies have indicated that the hippocampi of men and women differ anatomically, neurochemically, and also in degree of long-term potentiation.[19] Such evidence indicates that sex may influence the role of the hippocampus in learning. By contrast, a 2016 meta-analysis indicated that the hippocampus does not differ in volume between men and women.[20] One experiment examined the effects of stress on Pavlovian conditioning performance in both sexes and found that males' performance under stress was enhanced while female performance was impaired. Activation of the hippocampus is more dominant on the left side of hippocampus in females, while it is more dominant on the right side in males. This in turn influences cognitive reasoning; women use more verbal strategies than men when performing a task that requires cognitive thinking.[21] The hippocampus's relationship with other structures in the brain influences learning and has been found to be sexually dimorphic as well.[1]
Estradiol has been found to influence hippocampal development. Studies have shown neurogenesis, or the formation of new neurons, to be higher in the male hippocampus than in that of the female. This may be due to the lower levels of estradiol in the male brain compared to the female brain, providing a more optimal environment for neurogenesis.[22]
Frontal lobe
The ventromedial prefrontal cortex (VMPC) plays a key role in social emotional processing. In accordance with the sexual dimorphism of the amygdala, the right VMPC is more dominant in an active limbic system for males while the left is more dominant in females. These differences carry out to a behavioral level. For example, Koscik et al. wrote:
"A man with a unilateral right VMPC lesion, who was well educated and had worked successfully as a minister, was entirely unable to return to any form of gainful employment after his brain damage. He requires supervision for daily tasks and demonstrates severe disturbances in behavior and emotional regulation, including impulsivity and poor judgment. By contrast, a man with a unilateral left VMPC lesion was able to return to his job at a grain elevator and remains successfully employed there. He is remarkably free of disturbances to his social life and emotional functioning"
Orbital prefrontal cortex
Positron emission tomography studies have shown that men and women ranging from the ages of 19 to 32 years old metabolize glucose at significantly different rates in the orbital prefrontal cortex. Infant males who exhibited lesions on their orbital prefrontal cortex struggled with object reversal experiments, but females exhibiting such lesions did not have impaired performance in object reversal.[23]
Other regions and not region-specific
There are sex differences in locus coeruleus dendritic structure that allow for an increased reception and processing of limbic information in females compared to males.[18]
Aggressive and defiant behavior is also associated with decreased right anterior cingulate cortex (ACC) volume in boys.[18]
According to the neuroscience journal review series Progress in Brain Research, it has been found that males have larger and longer planum temporale and Sylvian fissure while females have significantly larger proportionate volumes to total brain volume in the superior temporal cortex, Broca's area, the hippocampus and the caudate.[24] The midsagittal and fiber numbers in the anterior commissure that connect the temporal poles and mass intermedia that connects the thalami is also larger in females.[24]
The journal review also found that male also have larger brain volume which can partly be accounted big bigger male body size. Researchers also found greater cortical thickness, cortical complexity and cortical surface area after adjusting for brain volume.[24] Given that cortical complexity and cortical features are positively correlated with intelligence, researchers postulated that these differences might have evolved for females to compensate for smaller brain size and equalize overall cognitive abilities with males.[24]
White/grey matter
Global and regional grey matter (GM) differs in men and women. Women have larger left orbitofrontal GM volumes and overall cortical thickness than men.[25] Behavioral implications of the greater volume have not yet been discovered. Women have a higher percentage of GM, whereas men have a higher percentage of white matter (WM) and of CSF (cerebrospinal fluid). In men the percentage of GM was higher in the left hemisphere, the percentage of WM was symmetric, and the percentage of CSF was higher in the right. Women showed no asymmetries. Both GM and WM volumes correlated moderately with global, verbal, and spatial performance across groups. However, the regression of cognitive performance and WM volume was significantly steeper in women.[26]
In a 2013 meta-analysis, researchers found on average males had larger grey matter volume in bilateral amygdalae, hippocampi, anterior parahippocampal gyri, posterior cingulate gyri, precuneus, putamen and temporal poles, areas in the left posterior and anterior cingulate gyri, and areas in the cerebellum bilateral VIIb, VIIIa and Crus I lobes, left VI and right Crus II lobes.[27] On the other hand, females on average had larger grey matter volume at the right frontal pole, inferior and middle frontal gyri, pars triangularis, planum temporale/parietal operculum, anterior cingulate gyrus, insular cortex, and Heschl's gyrus; bilateral thalami and precuneus; the left parahippocampal gyrus and lateral occipital cortex (superior division).[27] The meta-analysis found larger volumes in females were most pronounced in areas in the right hemisphere related to language in addition to several limbic structures such as the right insular cortex and anterior cingulate gyrus.[27]
Amber Ruigrok's 2013 meta-analysis also found greater grey matter density in the average male left amygdala, hippocampus, insula, pallidum, putamen, claustrum and right cerebellum.[27] The meta-analysis also found greater grey matter density in the average female left frontal pole[27]
Brain networks
A 2014 meta-analysis by researcher Ashley C.Hill found that although men and women commonly used the same brain networks for working memory, specific regions were gender specific.[28] For example, both men and women's active working memory networks composed of bilateral middle frontal gyri, left cingulate gyrus, right precuneus, left inferior and superior parietal lobes, right claustrum, and left middle temporal gyrus but women also tended have consistent activity in the limbic regions such as the anterior cingulate, bilateral amygdala and right hippocampus while men tended to have a distributed networks spread out among the cerebellum, portions of the superior parietal lobe, the left insula and bilateral thalamus.[28] In the work of[29] the authors have computed structural connectomes of 96 subjects of the Human Connectome Project, and they have proven that in numerous graph-theoretical parameters, the structural connectome of women are significantly better connected than the connectome of men. For example, women's connectome has more edges, higher minimum bipartition width, larger eigengap, greater minimum vertex cover than that of men. The minimum bipartition width (or the minimum balanced cut (graph theory)) is well-known measure of quality of computer multistage interconnection networks, it describes the possible bottlenecks in network communication: The higher this value is, the better is the network. The larger eigengap shows that the female connectome is better expander graph than the connectome of males. The better expanding property, the higher minimum bipartition width and the greater minimum vertex cover show deep advantages in network connectivity in the case of female braingraph.
Brain differences between homo- and heterosexuals
Brain wiring comparisons of homosexuals and persons of the opposite sex show that homosexuals may be born with a predisposition to be homosexual. Research at the Stockholm Brain Institute in Sweden found that homosexual men and heterosexual women have similar brain characteristics. Specifically, these similarities are in the overall size of the brain and the activity of the amygdala. The same is for heterosexual men and homosexual women. Molecular biologist at the National Institutes of Health, Dean Hamer, says, "this is from a series of observations showing there's a biological reason for sexual orientation".[30]
Ivanka Savic – Berglund conducted a study in which MRIs were used to measure the volume and shapes of the brain. She also used PET scans to view blood flow to the amygdala. Savic – Berglund found that in homosexual men and heterosexual women, the blood flowed to areas involved in fear and anxiety, whereas in heterosexual men and homosexual women, it tended to flow to pockets linked to aggression. When looking at hemisphere differences, the right hemisphere was found to be slightly larger than the left in heterosexual men and homosexual women, whereas those of homosexual men and heterosexual women were more symmetrical.[31]
Research has indicated that the corpus callosum is larger in homosexual men than in heterosexual men. This is significant because the corpus callosum is a structure that is developed early. In the Journal Science Simon LeVay showed that the third interstitial nucleus of the hypothalamus has neurons that are packed more together in homosexual men than in heterosexual men.[32] Connections from the amygdala to other parts of the brain are similar between homosexuals and persons of the opposite gender as shown through PET and MRI scans. For example, in homosexual men and heterosexual women, there were more connections from the left amygdala. In homosexual women and heterosexual men, there were more connections from the right amygdala. LeVay's results were not replicated in other studies. A 2001 study that attempted to replicate the findings concluded that "Although there was a trend for INAH3 to occupy a smaller volume in homosexual men than in heterosexual men, there was no difference in the number of neurons within the nucleus based on sexual orientation."[33]
Neurochemical differences
Hormones
Steroid hormones have several effects on brain development as well as maintenance of homeostasis throughout adulthood. One effect they exhibit is on the hypothalamus, where they increase synapse formation.[34] Estrogen receptors have been found in the hypothalamus, pituitary gland, hippocampus, and frontal cortex, indicating the estrogen plays a role in brain development. Gonadal hormone receptors have also been found in the basal forebrain nuclei.[35]
Estrogen and the female brain
Estradiol influences cognitive function, specifically by enhancing learning and memory in a dose-sensitive manner. Too much estrogen can have negative effects by weakening performance of learned tasks as well as hindering performance of memory tasks; this can result in females exhibiting poorer performance of such tasks when compared to males.[36]
It has been suggested that during development, estrogen can exhibit both feminizing and defeminizing effects on the human brain; high levels of estrogen induce male neural traits to develop while moderate levels induce female traits. In females, defeminizing effects are resisted because of the presence of α-fetoprotein (AFP), a carrier protein proposed to transport estrogen into brain cells, allowing the female brain to properly develop. The role of AFP is significant at crucial stages of development, however. Prenatally, AFP blocks estrogen. Postnatally, AFP decreases to ineffective levels; therefore, it is probable that estrogen exhibits its effects on female brain development postnatally.[37]
Ovariectomies, surgeries inducing menopause, or natural menopause cause fluctuating and decreased estrogen levels in women. This in turn can "attenuate the effects" of endogenous opioid peptides. Opioid peptides are known to play a role in emotion and motivation. β-endorphin (β-EP), an endogenous opioid peptide, content has been found to decrease (in varying amounts/brain region), post ovariectomy, in female rats within the hypothalamus, hippocampus, and pituitary gland. Such a change in β-EP levels could be the cause of mood swings, behavioral disturbances, and hot flashes in post menopausal women.[35]
Testosterone and the male brain
Testosterone has been found to play a big role during development but may have independent effects on sexually dimorphic brain regions in adulthood. Studies have shown that the medial amygdala of male hamsters exhibits lateralization and sexual dimorphism prior to puberty. Furthermore, organization of this structure during development is influenced by the presence of androgens and testosterone. This is evident when comparing medial amygdala volume of male and female rats, adult male brains have a medial amygdala of greater volume than do adult female brains which is partially due to androgen circulation.[38] It also heavily influences male development; a study found that perinatal females introduced to elevated testosterone levels exhibited male behavior patterns. In the absence of testosterone, female behavior is retained.[34] Testosterone's influence on the brain is caused by organizational developmental effects. It has been shown to influence proaptotic proteins so that they increase neuronal cell death in certain brain regions. Another way testosterone affects brain development is by aiding in the construction of the "limbic hypothalamic neural networks".[34]
Similar to how estrogen enhances memory and learning in women, testosterone has been found to enhance memory recall in men. In a study testing a correlation between memory a recall and testosterone levels in men, "fMRI analysis revealed that higher testosterone levels were related to increased brain activation in the amygdala during encoding of neutral pictures".[39]
Oxytocin and Vasopressin
Oxytocin is positively correlated with maternal behaviours, social recognition, social contact, sexual behaviour and pair bonding. Oxytocin appears at higher levels in women than in men.[40] Vasopressin on the other hand is more present in men and mediates sexual behavior, aggression and other social functions.[40][41]
Neurotransmitters
Whole level 5-HT serotonin levels are higher in women versus men while men synthesize serotonin significantly faster than women. Healthy women also have higher 5-HT transport availability in the diencephalon and brainstem areas of the brain.[42] Dopamine function is also increased in women especially dopamine transporter which regulates the availability of receptors. Women before the onset of menopause synthesize higher levels of striatal presynaptic dopamine than age-matched men.[42] Other neurotransmitters like μ-opioids show significantly higher binding potential in the cerebellum, amygdala and the thalamus for women than it does so for men.[43] Women are also more dependent on norepinephrine in the formation of long term emotional memories than men are.[43]
Male vs. female brain functionality
Neural masculinization is a developmental process where different sex hormones assist in the expression of male behavior.[44]
Stress

Stress has been found to induce an increase in serotonin, norepinephrine, and dopamine levels within the basolateral amygdala of male rats, but not within that of female rats. Furthermore, object recognition is impaired in males as a result of short term stress exposure. Neurochemical levels in the brain can change under the influence of stress exposure, particularly in regions associated with spatial and non-spatial memory, such as the prefrontal cortex and the hippocampus. Dopamine metabolite levels decrease post stress in male rats' brains, specifically within the CA1 region of the hippocampus.[45]
In female rats, both short term (1 hour) and long term (21 days) stress has been found to actually enhance spatial memory. Under stress, male rats exhibit deleterious effects on spatial memory, however female rats show a degree of resistance to this phenomenon. Stressed female rats' norepinephrine (NE) levels go up by about 50% in their prefrontal cortex while that of male rats goes down 50%.[45]
Cognitive tasks
It was once thought that sex differences in cognitive task and problem solving did not occur until puberty. However, new evidence now suggests that cognitive and skill differences are present earlier in development. For example, researchers have found that three- and four-year-old boys were better at targeting and at mentally rotating figures within a clock face than girls of the same age were. Prepubescent girls, however, excelled at recalling lists of words. These sex differences in cognition correspond to patterns of ability rather than overall intelligence. Laboratory settings are used to systematically study the sexual dimorphism in problem solving task performed by adults.[46]
On average, males excel relative to females at certain spatial tasks. Specifically, males have an advantage in tests that require the mental rotation or manipulation of an object.[47] They tend to outperform females in mathematical reasoning and navigation. In a computer simulation of a maze task, males completed the task faster and with fewer errors than their female counterparts. Additionally, males have displayed higher accuracy in tests of targeted motor skills, such as guiding projectiles.[46] Males are also faster on reaction time and finger tapping tests.[48]
On average, females excel relative to males on tests that measure recollection. They have an advantage on processing speed involving letters,digits and rapid naming tasks.[48] Females tend to have better object location memory and verbal memory.[49] They also perform better at verbal learning.[50] Females have better performance at matching items and precision tasks, such as placing pegs into designated holes. In maze and path completion tasks, males learn the goal route in fewer trials than females, but females remember more of the landmarks presented. This shows that females use landmarks in everyday situations to orient themselves more than males. Females are better at remembering whether objects had switched places or not.[46]
Studies using the Iowa gambling task, or Iowa Card Task, have examined cognitive reasoning and decision-making in males and females. A study in which participants of various age groups who were asked to perform the Iowa Card Task produced data showing that males and females differ in their decision making processes on the neurological level. The study suggests that decision-making in females may be guided by avoidance of negativity while decision making in males is mainly guided by assessing the long term outcome of a situation. They also found that males outperformed females in the Iowa Card Task, but there was a negative correlation between elevated testosterone levels and performance in the card task which indicates gonadal hormones influence decision-making.[23]
See also
References
- 1 2 3 4 Cahill L (June 2006). "Why sex matters for neuroscience". Nature Reviews. Neuroscience. 7 (6): 477–84. PMID 16688123. doi:10.1038/nrn1909.
- ↑ Ruigrok, Amber N.V.; Salimi-Khorshidi, Gholamreza; Lai, Meng-Chuan; Baron-Cohen, Simon; Lombardo, Michael V.; Tait, Roger J.; Suckling, John (2014). "A meta-analysis of sex differences in human brain structure". Neuroscience & Biobehavioral Reviews. 39: 34–50. ISSN 0149-7634. PMC 3969295
 . PMID 24374381. doi:10.1016/j.neubiorev.2013.12.004.
. PMID 24374381. doi:10.1016/j.neubiorev.2013.12.004. - ↑ Daphna Joela; Zohar Berman; Ido Tavor; Nadav Wexler; Olga Gaber; Yaniv Stein; Nisan Shefia; Jared Pool; Sebastian Urchse; Daniel S. Marguliese; Franziskus Lieme; Jürgen Hänggif; Lutz Jänckef; Yaniv Assaf (October 23, 2015). "Sex beyond the genitalia: The human brain mosaic". Proceedings of the National Academy of Sciences of the United States of America. 112 (50): 15468–15473. PMC 4687544
 . PMID 26621705. doi:10.1073/pnas.1509654112.
. PMID 26621705. doi:10.1073/pnas.1509654112. - ↑ Eyal Abraham; Talma Hendler; Irit Shapira-Lichter; Yaniv Kanat-Maymon; Orna Zagoory-Sharon; Ruth Feldman (May 2014). "Father's brain is sensitive to childcare experiences". Proceedings of the National Academy of Sciences. 111 (27): 9792–9797. PMC 4103311
 . PMID 24912146. doi:10.1073/pnas.1402569111.
. PMID 24912146. doi:10.1073/pnas.1402569111. - ↑ Jeffrey Derks; Lydia Krabbendam (2013). "Is the Brain the Key to a Better Understanding of Gender Differences in the Classroom?". International Journal of Gender, Science and Technology. 5 (3).
- ↑ Swaab DF, Hofman MA (1984). "Sexual differentiation of the human brain. A historical perspective". Progress in Brain Research. Progress in Brain Research. 61: 361–74. ISBN 9780444805324. PMID 6396708. doi:10.1016/S0079-6123(08)64447-7.
- ↑ Hofman MA, Swaab DF (1991). "Sexual dimorphism of the human brain: myth and reality". Experimental and Clinical Endocrinology. 98 (2): 161–70. PMID 1778230. doi:10.1055/s-0029-1211113.
- ↑ McCarthy, Margaret M. (2016-02-19). "Philosophical Transactions of the Royal Society B: Biological Sciences: 371 (1688)". Phil. Trans. R. Soc. B. Theme issue ‘Multifaceted origins of sex differences in the brain’. 371 (1688). ISSN 0962-8436.
- ↑ Jacobs LF (February 1996). "Sexual selection and the brain". Trends in Ecology & Evolution. 11 (2): 82–6. PMID 21237767. doi:10.1016/0169-5347(96)81048-2.
- ↑ Kansaku K, Yamaura A, Kitazawa S (September 2000). "Sex differences in lateralization revealed in the posterior language areas". Cerebral Cortex. 10 (9): 866–72. PMID 10982747. doi:10.1093/cercor/10.9.866.
- ↑ Shaywitz BA, Shaywitz SE, Pugh KR, et al. (February 1995). "Sex differences in the functional organization of the brain for language". Nature. 373 (6515): 607–9. PMID 7854416. doi:10.1038/373607a0.
- ↑ Sommer IE, Aleman A, Somers M, Boks MP, Kahn RS (April 2008). "Sex differences in handedness, asymmetry of the planum temporale and functional language lateralization". Brain Research. 1206: 76–88. PMID 18359009. doi:10.1016/j.brainres.2008.01.003.
- 1 2 3 Ingalhalikar M, Smith A, Parker D, et al. (January 2014). "Sex differences in the structural connectome of the human brain". Proceedings of the National Academy of Sciences of the United States of America. 111 (2): 823–8. PMC 3896179
 . PMID 24297904. doi:10.1073/pnas.1316909110. Lay summary – ScienceDaily (December 2, 2013).
. PMID 24297904. doi:10.1073/pnas.1316909110. Lay summary – ScienceDaily (December 2, 2013). - ↑ Joseph M. Andreano, Bradford C. Dickerson, and Lisa Feldman Barrett Sex differences in the persistence of the amygdala response to negative material, 2013)
- ↑ Marwha, Dhruv; Halari, Meha; Eliot, Lise (2017-02-15). "Meta-analysis reveals a lack of sexual dimorphism in human amygdala volume". NeuroImage. 147: 282–294. doi:10.1016/j.neuroimage.2016.12.021.
- ↑ Foland-Ross, LC; Altshuler, LL; Bookheimer, SY; et al. (2010). "Amygdala reactivity in healthy adults is correlated with prefrontal cortical thickness". Journal of Neuroscience. 30 (49): 16673–16678. PMC 3046875
 . PMID 21148006. doi:10.1523/JNEUROSCI.4578-09.2010.
. PMID 21148006. doi:10.1523/JNEUROSCI.4578-09.2010. - ↑ Markowitsch, H. (1998). Differential contribution of right and left amygdala to affective information processing. IOS Press. 11(4), 233–244.
- 1 2 3 Kret, M.E. (2011). "A review on sex differences in processing emotional signals". Neuropsychologia Review. 50 (7): 1211–1221. doi:10.1016/j.neuropsychologia.2011.12.022. Retrieved 2012. Check date values in:
|access-date=(help) - ↑ Maren, Stephen; De Oca, Beatrice; Fanselow, Michael S. (1994-10-24). "Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning". Brain Research. 661 (1–2): 25–34. doi:10.1016/0006-8993(94)91176-2.
- ↑ Tan, Anh; Ma, Wenli; Vira, Amit; Marwha, Dhruv; Eliot, Lise (2016-01-01). "The human hippocampus is not sexually-dimorphic: Meta-analysis of structural MRI volumes". NeuroImage. 124: 350–366. doi:10.1016/j.neuroimage.2015.08.050.
- ↑ Frings L, Wagner K, Unterrainer J, Spreer J, Halsband U, Schulze-Bonhage A (March 2006). "Gender-related differences in lateralization of hippocampal activation and cognitive strategy". NeuroReport. 17 (4): 417–21. PMID 16514369. doi:10.1097/01.wnr.0000203623.02082.e3.
- ↑ Bowers JM, Waddell J, McCarthy MM (2010). "A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol". Biology of Sex Differences. 1 (1): 8. PMC 3016241
 . PMID 21208470. doi:10.1186/2042-6410-1-8.
. PMID 21208470. doi:10.1186/2042-6410-1-8. - 1 2 Overman WH (June 2004). "Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex". Brain and Cognition. 55 (1): 134–47. PMID 15134848. doi:10.1016/S0278-2626(03)00279-3.
- 1 2 3 4 Sex difference in the human brain, their underpinnings and implications. Elsevier. 2010-12-03. ISBN 9780444536310.
- ↑ Luders E, Gaser C, Narr KL, Toga AW (November 2009). "Why sex matters: brain size independent differences in gray matter distributions between men and women". The Journal of Neuroscience. 29 (45): 14265–70. PMC 3110817
 . PMID 19906974. doi:10.1523/JNEUROSCI.2261-09.2009.
. PMID 19906974. doi:10.1523/JNEUROSCI.2261-09.2009. - ↑ "Sex Differences in Brain Gray and White Matter in Healthy Young Adults: Correlations with Cognitive Performance".
- 1 2 3 4 5 Ruigrok, Amber N. V.; Salimi-Khorshidi, Gholamreza; Lai, Meng-Chuan; Baron-Cohen, Simon; Lombardo, Michael V.; Tait, Roger J.; Suckling, John (2014-02-01). "A meta-analysis of sex differences in human brain structure". Neuroscience & Biobehavioral Reviews. 39: 34–50. PMC 3969295
 . PMID 24374381. doi:10.1016/j.neubiorev.2013.12.004.
. PMID 24374381. doi:10.1016/j.neubiorev.2013.12.004. - 1 2 Hill, Ashley C. (2014). "Gender differences in working memory networks: A BrainMap meta-analysis" (PDF). Biological Psychology. 102: 18–29. PMC 4157091
 . PMID 25042764. doi:10.1016/j.biopsycho.2014.06.008.
. PMID 25042764. doi:10.1016/j.biopsycho.2014.06.008. - ↑ Szalkai, Balazs; et al. (2015). "Graph Theoretical Analysis Reveals: Women’s Brains Are Better Connected than Men's". PLoS ONE. 10 (7): e0130045. PMC 4488527
 . PMID 26132764. doi:10.1371/journal.pone.0130045.
. PMID 26132764. doi:10.1371/journal.pone.0130045. - ↑ Swaminathan, Nikhil. "Study Says Brains of Gay Men and Women Are erSimilar". Scientific American, a division of Nature America, Inc.
- ↑ Owen, James. "Gay Men, Straight Women Have Similar Brains". National Geographic News.
- ↑ Levay, S (August 30, 1991). "A difference in hypothalamic structure between heterosexual and homosexual men". Science. 5023. 253 (5023): 1034–1037. PMID 1887219. doi:10.1126/science.1887219.
- ↑ Byne, William; Tobet, Stuart; Mattiace, Linda A.; Lasco, Mitchell S.; Kemether, Eileen; Edgar, Mark A.; Morgello, Susan; Buchsbaum, Monte S.; Jones, Liesl B. (2001-09-01). "The Interstitial Nuclei of the Human Anterior Hypothalamus: An Investigation of Variation with Sex, Sexual Orientation, and HIV Status". Hormones and Behavior. 40 (2): 86–92. PMID 11534967. doi:10.1006/hbeh.2001.1680.
- 1 2 3 Simerly RB (February 2005). "Wired on hormones: endocrine regulation of hypothalamic development". Current Opinion in Neurobiology. 15 (1): 81–5. PMID 15721748. doi:10.1016/j.conb.2005.01.013.
- 1 2 Genazzani AR, Pluchino N, Luisi S, Luisi M (2007). "Estrogen, cognition and female ageing". Human Reproduction Update. 13 (2): 175–87. PMID 17135285. doi:10.1093/humupd/dml042.
- ↑ Korol DL (November 2004). "Role of estrogen in balancing contributions from multiple memory systems". Neurobiology of Learning and Memory. 82 (3): 309–23. PMID 15464412. doi:10.1016/j.nlm.2004.07.006.
- ↑ Bakker J, Baum MJ (January 2008). "Role for estradiol in female-typical brain and behavioral sexual differentiation". Frontiers in Neuroendocrinology. 29 (1): 1–16. PMC 2373265
 . PMID 17720235. doi:10.1016/j.yfrne.2007.06.001.
. PMID 17720235. doi:10.1016/j.yfrne.2007.06.001. - ↑ Cooke BM, Woolley CS (November 2005). "Sexually dimorphic synaptic organization of the medial amygdala". The Journal of Neuroscience. 25 (46): 10759–67. PMID 16291949. doi:10.1523/JNEUROSCI.2919-05.2005.
- ↑ Ackermann S, Spalek K, Rasch B, et al. (September 2012). "Testosterone levels in healthy men are related to amygdala reactivity and memory performance". Psychoneuroendocrinology. 37 (9): 1417–24. PMID 22341731. doi:10.1016/j.psyneuen.2012.01.008.
- 1 2 Carter, C.Sue (2006). "Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders?" (PDF). Behavioural Brain Research. 176 (1): 170–186. PMID 17000015. doi:10.1016/j.bbr.2006.08.025.
- ↑ Skuse, David H. (2006-11-01). "Sexual dimorphism in cognition and behaviour: the role of X-linked genes". European Journal of Endocrinology. 155 (suppl 1): S99–S106. ISSN 0804-4643. doi:10.1530/eje.1.02263.
- 1 2 Cosgrove, Kelly P.; Mazure, Carolyn M.; Staley, Julie K. (2007-10-15). "Evolving Knowledge of Sex Differences in Brain Structure, Function and Chemistry". Biological Psychiatry. 62 (8): 847–855. ISSN 0006-3223. PMC 2711771
 . PMID 17544382. doi:10.1016/j.biopsych.2007.03.001.
. PMID 17544382. doi:10.1016/j.biopsych.2007.03.001. - 1 2 Ngun, Tuck C; Ghahramani, Negar; Sánchez, Francisco J.; Bocklandt, Sven; Vilain, Eric (2011-04-01). "The Genetics of Sex Differences in Brain and Behavior". Frontiers in neuroendocrinology. 32 (2): 227–246. ISSN 0091-3022. PMC 3030621
 . PMID 20951723. doi:10.1016/j.yfrne.2010.10.001.
. PMID 20951723. doi:10.1016/j.yfrne.2010.10.001. - ↑ Wu MV, Manoli DS, Fraser EJ, et al. (October 2009). "Estrogen masculinizes neural pathways and sex-specific behaviors". Cell. 139 (1): 61–72. PMC 2851224
 . PMID 19804754. doi:10.1016/j.cell.2009.07.036.
. PMID 19804754. doi:10.1016/j.cell.2009.07.036. - 1 2 Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN (April 2009). "Sex-dependent changes in anxiety, memory, and monoamines following one week of stress". Physiology & Behavior. 97 (1): 21–9. PMID 19419681. doi:10.1016/j.physbeh.2009.01.012.
- 1 2 3 Kimura, Doreen (July 31, 2000). Sex and Cognition. A Bradford Book. p. 28. ISBN 0262611643.
- ↑ Miller Halpern, David Diane (2013). "The New Science of Cognitive Sex Differences review". Trends in Cognitive Science. 18: 37–45. doi:10.1016/j.tics.2013.10.011.
- 1 2 Roivainen, Eka (2011). "Gender differences in processing speed: A review of recent research". Learning and Individual Differences. 21 (2): 145–149. doi:10.1016/j.lindif.2010.11.021.
- ↑ Li, Rena (2014-09-01). "Why women see differently from the way men see? A review of sex differences in cognition and sports". Journal of Sport and Health Science. 3 (3): 155–162. PMC 4266559
 . PMID 25520851. doi:10.1016/j.jshs.2014.03.012.
. PMID 25520851. doi:10.1016/j.jshs.2014.03.012. - ↑ Wallentin, Mikkel (2009). "Putative sex differences in verbal abilities and language cortex: A critical review". Brain and Language. 108 (3): 175–183. PMID 18722007. doi:10.1016/j.bandl.2008.07.001.