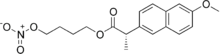
Naproxcinod
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| Synonyms | AZD-3582, HCT-3012 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C18H21NO6 |
| Molar mass | 347.362 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Naproxcinod (nitronaproxen) is a nonsteroidal anti-inflammatory drug (NSAID) developed by the French pharmaceutical company NicOx. It is a derivative of naproxen with a nitroxybutyl ester to allow it to also act as a nitric oxide (NO) donor. This second mechanism of action makes naproxcinod the first in a new class of drugs, the cyclooxygenase inhibiting nitric oxide donators (CINODs), that are hoped to produce similar analgesic efficacy to traditional NSAIDs, but with less gastrointestinal and cardiovascular side effects.[1][2]
On December 2006, Scientific American distinguished naproxcinod as one of the ten most promising treatments for the world's biggest health threats;[3] however, in 2010 the U.S. Food and Drug Administration determined that further clinical trials would be needed to obtain approval.[4]
Current situation in pain treatment
Many people are currently relying on traditional NSAIDs and COX-2 inhibitors (for example celecoxib) to treat chronic pain and inflammation. COX-2 inhibitors have been associated with an increased risk of serious cardiovascular events such as strokes or heart attacks.[5] Therefore, there is an unmet need for safer medications. This need is particularly acute among patients with high cardiovascular risk like hypertension which represents 50% of osteoarthritis sufferers.
Indications
Three phase III clinical trials led by NicOx have shown that naproxcinod was effective to treat pain against knee osteoarthritis[6][7][8] and hip osteoarthrtis.[9] A phase II study showed no significant differences in efficacy between naproxcinod and the COX-2 inhibitor rofecoxib in the treatment of pain.[10]
In osteoarthritis, a 750 mg dose is equipotent to 500 mg of naproxen for the treatment of inflammation but with the added benefit of attenuating the cardiovascular effects traditionally associated with NSAIDs.[11]
In July 2010 the FDA decided not to approve naproxcinod.[4]
Mechanism of action
Naproxcinoid is metabolized to naproxen and a nitric oxide donating moiety. NO has various cardiovascular effects, including vasodilatory and platelet-inhibitory actions as well as the inhibition of vascular smooth muscle proliferation that serves to maintain normal vascular tone.[11]
Safety profile
Blood pressure profile
According to some experts, cardiovascular risks induced by COX-2 inhibitors are caused by increases in blood pressure. Naproxcinod demonstrated in a clinical trial with 916 patients to have a blood pressure profile similar to placebo.[11] Two phase II randomized controlled trials have shown a decreased systolic blood pressure by 2.1 mmHg after patients took naproxcinod (375 mg or 750 mg twice daily) for six weeks. These effects were especially pronounced in hypertensive populations.[10][12]
Clinical relevance of small increase in blood pressure
During an U.S. Food and Drug Administration (FDA) COX-2 advisory committee meeting, doctors have underlined the important role of small increase in blood pressure.[13] They cited the CAMELOT trial which has concluded that even a small decrease in systolic blood pressure of 5 mmHg could lead to a reduction of 31% in cardiovascular events.[14] Clinical studies about rofecoxib have shown that this drug increases the systolic blood pressure.[15]
A 2005 analysis shows that a blood pressure decrease of 3.1 mmHG could avoid over 30,000 deaths from stroke and 2,000 deaths from coronary disease, resulting in more than 449,000 person years of life saved and 1.4 billion US$ in direct health care cost savings.[16]
Gastrointestinal safety
NSAIDs have also been associated with gastrointestinal risks such as bleedings. Early studies demonstrated that naproxcinod had a better gastrointestinal profile than naproxen, especially for the gastroduodenal mucosa,[17][18] but a 2009 review has found only a slight and possibly not clinically relevant reduction of gastrointestinal side-effects.[19][20]
Contraindications and adverse effects
Similarly to NSAIDs, adverse effects of naproxcinod include gastrointestinal bleedings.[19][20]
Commercialization
Naproxcinod completed a phase III study needed for a New Drug Application (NDA). As a result, Nicox submitted its project to the FDA in September 2009.[21] In July 2010, the FDA decided not to approve naproxcinod without further clinical trials.[4] Nicox submitted a Marketing Authorization Application (MAA) to the European Medicines Agency (EMEA) in December 2009.[22] Nicox and Fera Pharmaceuticals announced in November 2015 that they had entered into a license agreement for the development and commercialization of naproxcinod in the United States.[23]
See also
- Nitrosoprodenafil – a dual PDE5 inhibitor / NO donor
References
- ↑ Ellis, JL; Augustyniak, ME; Cochran, ED; Earl, RA; Garvey, DS; Gordon, LJ; Janero, DR; Khanapure, SP; et al. (2005). "NMI-1182, a gastro-protective cyclo-oxygenase-inhibiting nitric oxide donor". Inflammopharmacology. 12 (5–6): 521–34. PMID 16259719. doi:10.1163/156856005774382661.
- ↑ Cirino, G; Distrutti, E; Wallace, JL (2006). "Nitric oxide and inflammation". Inflammation & allergy drug targets. 5 (2): 115–9. PMID 16613570. doi:10.2174/187152806776383143.
- ↑ Special Report: 10 Promising Treatments for World's Biggest Health Threats, By Charles Q. Choi. 2006
- 1 2 3 NicOx Shares Plummet as FDA Says Osteoarthritis Drug Not Ready for Approval
- ↑ Baron, JA; Sandler, RS; Bresalier, RS; Lanas, A; Morton, DG; Riddell, R; Iverson, ER; Demets, DL (2008). "Cardiovascular events associated with rofecoxib: final analysis of the APPROVe trial". Lancet. 372 (9651): 1756–64. PMID 18922570. doi:10.1016/S0140-6736(08)61490-7.
- ↑ "NicOx Announces Top-Line Results From Naproxcinod 52-Week 301 Safety Extension". 24 July 2008. Retrieved 2 February 2010.
- ↑ Clinical trial number NCT00542555 for "Analgesic Efficacy and Safety Study of Naproxcinod in Subjects With Osteoarthritis of the Knee" at ClinicalTrials.gov
- ↑ Clinical trial number NCT00504127 for "Efficacy and Safety Study of Naproxcinod in Subjects With Osteoarthritis of the Knee" at ClinicalTrials.gov
- ↑ Clinical trial number NCT00541489 for "Efficacy and Safety Study of Naproxcinod in Subjects With Osteoarthritis of the Hip" at ClinicalTrials.gov
- 1 2 Karlsson, J; Pivodic, A; Aguirre, D; Schnitzer, TJ (2009). "Efficacy, safety, and tolerability of the cyclooxygenase-inhibiting nitric oxide donator naproxcinod in treating osteoarthritis of the hip or knee". The Journal of rheumatology. 36 (6): 1290–7. PMID 19411388. doi:10.3899/jrheum.081011.
- 1 2 3 White WB, Schnitzer TJ, Fleming R, Duquesroix B, Beekman M (September 2009). "Effects of the cyclooxygenase inhibiting nitric oxide donator naproxcinod versus naproxen on systemic blood pressure in patients with osteoarthritis". Am. J. Cardiol. 104 (6): 840–5. PMID 19733721. doi:10.1016/j.amjcard.2009.05.014.
- ↑ Wallace, JL; Viappiani, S; Bolla, M (2009). "Cyclooxygenase-inhibiting nitric oxide donators for osteoarthritis". Trends in pharmacological sciences. 30 (3): 112–7. PMID 19230986. doi:10.1016/j.tips.2009.01.001.
- ↑ "Analysis of FDA COX-2 Advisory Committee Meeting" (PDF). 2005. p. 21.
- ↑ Nissen, Steven E; Tuzcu, EM; Libby, P; Thompson, PD; Ghali, M; Garza, D; Berman, L; Shi, H; et al. (2004). "Effect of Antihypertensive Agents on Cardiovascular Events in Patients With Coronary Disease and Normal Blood Pressure". JAMA. 292 (18): 2217–25. PMID 15536108. doi:10.1001/jama.292.18.2217.
- ↑ Whelton, A (2002). "COX-2-specific inhibitors and the kidney: effect on hypertension and oedema". Journal of Hypertension Supplement. 20 (6): S31–5. PMID 12683425.
- ↑ Grover, SA; Coupal, L; Zowall, H (2005). "Treating osteoarthritis with cyclooxygenase-2-specific inhibitors: what are the benefits of avoiding blood pressure destabilization?". Hypertension. 45 (1): 92–7. PMID 15545508. doi:10.1161/01.HYP.0000149684.01903.b8.
- ↑ Wilder-Smith, CH; Jonzon, B; Fornstedt-Wallin, B; Hedman, A; Karlsson, P (2006). "Dose-effect comparisons of the CINOD AZD3582 and naproxen on upper gastrointestinal tract mucosal injury in healthy subjects". Scandinavian journal of gastroenterology. 41 (3): 264–73. PMID 16497612. doi:10.1080/00365520510024197.
- ↑ Hawkey, CJ; Jones, JI; Atherton, CT; Skelly, MM; Bebb, JR; Fagerholm, U; Jonzon, B; Karlsson, P; Bjarnason, IT (2003). "Gastrointestinal safety of AZD3582, a cyclooxygenase inhibiting nitric oxide donator: proof of concept study in humans". Gut. 52 (11): 1537–42. PMC 1773862
 . PMID 14570719. doi:10.1136/gut.52.11.1537.
. PMID 14570719. doi:10.1136/gut.52.11.1537. - 1 2 Geusens, P (2009). "Naproxcinod, a new cyclooxygenase-inhibiting nitric oxide donator (CINOD)". Expert opinion on biological therapy. 9 (5): 649–57. PMID 19392579. doi:10.1517/14712590902926071.
- 1 2 Schubert-Zsilavecz, M, Wurglics, M, Neue Arzneimittel 2010
- ↑ Michelson, Marcel (25 September 2009). "NicOx submits naproxcinod application to FDA". Reuters. Retrieved 3 February 2010.
- ↑ "MAA for naproxcinod submitted to EMEA through centralized procedure". NewsMedical.net. 22 December 2009. Retrieved 2 February 2010.
- ↑ "Fera Pharmaceuticals - Press Releases". www.ferapharma.com. Retrieved 2016-04-20.