Nitrosamine
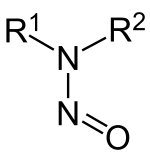
Nitrosamines are chemical compounds of the chemical structure R1N(–R2)–N=O, that is, a nitroso group bonded to an amine. Most nitrosamines are carcinogenic.
Uses
Nitrosamines are used in the manufacture of some cosmetics, pesticides, and in most rubber products.
Occurrence
Nitrosamines occur in latex products such as balloons,[1] and in many foods and other consumables. Nitrosamines from condoms are not thought to be of toxicological significance.[2]
In foods, nitrosamines are produced from nitrites and secondary amines, which often occur in the form of proteins. Their formation can occur only under certain conditions, including strongly acidic conditions such as that of the human stomach. High temperatures, as in frying, can also enhance the formation of nitrosamines. The presence of nitrosamines may be identified by the Liebermann nitroso reaction[3] (not to be confused with the Liebermann reagent which reacts red or blue in the presence of phenols[4]).
Under acidic conditions the nitrite forms nitrous acid (HNO2), which is protonated and splits into the nitrosonium cation N≡O+ and water:
- H
2NO+
2 → H2O + NO+.
The nitrosonium cation then reacts with an amine to produce nitrosamine.[5]
These processes lead to significant levels of nitrosamines in many foodstuffs, especially beer, fish, and fish byproducts, and also in meat and cheese products preserved with nitrite compounds. The U.S. government established limits on the amount of nitrites used in meat products in order to decrease cancer risk in the population.[6] There are also rules about adding ascorbic acid or related compounds to meat, as the compounds inhibit formation of nitrosamines.[7]
Tobacco-specific nitrosamines can also be found in tobacco smoke, American dip snuff, chewing tobacco, and to a much lesser degree, snus (127.9 ppm for American dip snuff compared to 2.8 ppm in Swedish snuff or snus).[8]
Nitrosamines can also be found in e-cigarette vapour, though this is reported to be trace amounts, possibly from the propylene glycol and/or vegetable glycerine used in manufacture.[9]
Nitrosamines can be formed from nitrates (usually agricultural runoff) in drinking water upon ingestion. The EPA standard is 10 ppm[10] but is considered by the National Academy of Sciences as lacking margin of safety for sensitive individuals.[11] This standard has not changed in over 40 years despite repeated warnings from the Academy.[11]
Biology
Cancer
In 1956, two British scientists, John Barnes and Peter Magee, reported that dimethylnitrosamine produced liver tumours in rats. Research was undertaken and approximately 90% of nitrosamine compounds were deemed to be carcinogenic.[12]
In the 1970s, an elevated frequency of liver cancer was found in Norwegian farm animals. The farm animals had been fed on herring meal, which was preserved using sodium nitrite. The sodium nitrite had reacted with dimethylamine in the fish and produced dimethylnitrosamine.[13] Nitrosamines can cause cancers in a wide variety of animal species, a feature that suggests that they may also be carcinogenic in humans. At present, available epidemiological evidence from case-control studies on nitrite and nitrosamine intake supports a positive association with gastric cancer risk. Regarding oesophageal cancer, available evidence supports a positive association between nitrite and nitrosamine intake and gastric cancer (GC), between meat and processed meat intake and GC and oesophageal cancer, and between preserved fish, vegetable and smoked food intake and GC, but is not conclusive.[14]
Hydrazines derived from these nitrosamines, e.g. UDMH, are also carcinogenic.
Inhibition
Endogenous nitrosamine formation can be inhibited by ascorbic acid.[15] In the case of formation of carcinogenic nitrosamines in the stomach from dietary nitrite (used as processed meat preservative), ascorbic acid markedly decreases nitrosamine formation in the absence of fat in the meal; but when 10% fat is present, this reverses the effect such that ascorbic acid then markedly increases nitrosamine formation.[16][17]
Examples
| Substance name | CAS number | Synonyms | Molecular formula | Physical appearance | Carcinogenicity category |
|---|---|---|---|---|---|
| N-Nitrosonornicotine | 16543-55-8 | NNN | C9H11N3O | Light yellow low-melting solid | |
| 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone[18] | 64091-91-4 | NNK, 4′-(nitrosomethylamino)-1-(3-pyridyl)-1-butanone | C10H15N3O2 | Light yellow oil | |
| N-Nitrosodimethylamine | 62-75-9 | Dimethylnitrosamine, N,N-dimethylnitrosamine, NDMA, DMN | C2H6N2O | Yellow liquid | EPA-B2; IARC-2A; OSHA carcinogen; TLV-A3 |
| N-Nitrosodiethylamine | 55-18-5 | Diethylnitrosamide, diethylnitrosamine, N,N-diethylnitrosamine, N-ethyl-N-nitrosoethanamine, diethylnitrosamine, DANA, DENA, DEN, NDEA | C4H10N2O | Yellow liquid | EPA-B2; IARC-2A |
| 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol | 76014-81-8 | NNAL | |||
| N-Nitrosoanabasine | 37620-20-5 | NAB | C10H13N3O | Yellow Oil | IARC-3 |
| N-Nitrosoanatabine | 71267-22-6 | NAT | C10H11N3O | Clear yellow-to-orange oil | IARC-3 |
See also
References
- ↑ Altkofer, Werner; Braune, Stefan; Ellendt, Kathi; Kettl-Grömminger, Margit; Steiner, Gabriele (2005). "Migration of nitrosamines from rubber products - are balloons and condoms harmful to the human health?". Molecular Nutrition & Food Research. 49 (3): 235–8. PMID 15672455. doi:10.1002/mnfr.200400050.
- ↑ Proksch, Ehrhardt (2001). "Review Toxicological evaluation of nitrosamines in condoms". International Journal of Hygiene and Environmental Health. 204 (2–3): 103–10. PMID 11759152. doi:10.1078/1438-4639-00087.
- ↑ Vogel, A. I. (1962). Practical Organic Chemistry (3rd ed.). Impression. p. 649.
- ↑ Glagovich, Neil. "The Libermann Nitroso Reaction". Connecticut State University. Archived from the original on March 5, 2013. Retrieved 28 December 2015.
- ↑ Vogel, A. I. (1962). Practical Organic Chemistry (3rd ed.). Impression. p. 1074.
- ↑ Honikel, Karl-Otto (2008). "The use and control of nitrate and nitrite for the processing of meat products". Meat Science. 78 (1–2): 68–76. PMID 22062097. doi:10.1016/j.meatsci.2007.05.030.
- ↑ Scanlan, Richard A. (22 April 2000). "Nitrosamines and Cancer". The Linus Pauling Institute. Retrieved 28 December 2015.
- ↑ Gregory N. Connolly; Howard Saxner (August 21, 2001). "Informational Update Research on Tobacco Specific Nitrosamines (TSNAs) in Oral Snuff and a Request to Tobacco Manufacturers to Voluntarily Set Tolerance Limits For TSNAs in Oral Snuff".
- ↑ "E-cigarettes: harmless inhaled or exhaled, No second hand smoke". Health New Zealand (an e-cigarette lobbyist site). Retrieved 27 June 2014.
- ↑ https://www.epa.gov/ground-water-and-drinking-water/table-regulated-drinking-water-contaminants
- 1 2 http://www.ewg.org/research/pouring-it/health-effects-nitrate-exposure
- ↑ Advances in Agronomy. Academic Press. 2013-01-08. pp. 159–. ISBN 978-0-12-407798-0.
- ↑ Joyce I. Boye; Yves Arcand (2012-01-10). Green Technologies in Food Production and Processing. Springer Science & Business Media. pp. 573–. ISBN 978-1-4614-1586-2.
- ↑ Jakszyn, P; Gonzalez, CA (2006). "Nitrosamine and related food intake and gastric and oesophageal cancer risk: A systematic review of the epidemiological evidence". World journal of gastroenterology. 12 (27): 4296–303. PMC 4087738
 . PMID 16865769. doi:10.3748/wjg.v12.i27.4296.
. PMID 16865769. doi:10.3748/wjg.v12.i27.4296. - ↑ Tannenbaum SR, Wishnok JS, Leaf CD (1991). "Inhibition of nitrosamine formation by ascorbic acid". The American Journal of Clinical Nutrition. 53 (1 Suppl): 247S–250S. Bibcode:1987NYASA.498..354T. PMID 1985394. doi:10.1111/j.1749-6632.1987.tb23774.x. Retrieved 2015-06-06.
Evidence now exists that ascorbic acid is a limiting factor in nitrosation reactions in people.
- ↑ Combet, E; Paterson, S; Iijima, K; Winter, J; Mullen, W; Crozier, A; Preston, T; McColl, K. E. (2007). "Fat transforms ascorbic acid from inhibiting to promoting acid-catalysed N-nitrosation". Gut. 56 (12): 1678–84. PMC 2095705
 . PMID 17785370. doi:10.1136/gut.2007.128587.
. PMID 17785370. doi:10.1136/gut.2007.128587. - ↑ Combet, E; El Mesmari, A; Preston, T; Crozier, A; McColl, K. E. (2010). "Dietary phenolic acids and ascorbic acid: Influence on acid-catalyzed nitrosative chemistry in the presence and absence of lipids". Free Radical Biology and Medicine. 48 (6): 763–71. PMID 20026204. doi:10.1016/j.freeradbiomed.2009.12.011.
- ↑ Hecht, Steven S.; Borukhova, Anna; Carmella, Steven G. "Tobacco specific nitrosamines" Chapter 7; of "Nicotine safety and toxicity" Society for Research on Nicotine and Tobacco; 1998 - 203 pages
External links
- Oregon State University, Linus Pauling Institute article on Nitrosamines and cancer, including info on history of meat laws
- Risk factors in Pancreatic Cancer