Murine polyomavirus
| Murine polyomavirus | |
|---|---|
 | |
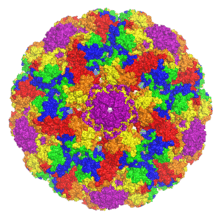
| The capsid protein VP1 assembled into an icosahedral capsid structure comprising 72 pentamers, colored by distance from the interior center. From PDB: 1SIE.[1] | |
| Virus classification | |
| Group: | Group I (dsDNA) |
| Family: | Polyomaviridae |
| Genus: | Alphapolyomavirus |
| Species: | Murine polyomavirus |
Murine polyomavirus (also known as mouse polyomavirus, Polyomavirus muris, or Mus musculus polyomavirus 1, and in older literature as SE polyoma or parotid tumor virus; abbreviated MPyV) is an unenveloped double-stranded DNA virus of the polyomavirus family. The first member of the family discovered, it was originally identified by accident in the 1950s.[2][3] A component of mouse leukemia extract capable of causing tumors, particularly in the parotid gland, in newborn mice was reported by Ludwik Gross in 1953[4] and identified as a virus by Sarah Stewart and Bernice Eddy at the National Cancer Institute, after whom it was once called "SE polyoma".[5][6][7] Stewart and Eddy would go on to study related polyomaviruses such as SV40 that infect primates, including humans. These discoveries were widely reported at the time and formed the early stages of understanding of oncoviruses.[8][9]
Pathology
MPyV is primarily spread among mice via the intranasal route and is shed in urine. Genetic susceptibility to MPyV infection among mice varies significantly, and not all MPyV strains are oncogenic.[7] In general, only newborns and immunosuppressed mice (usually transgenic) develop tumors upon infection; although originally observed as a cause of parotid gland tumors, the virus may induce solid tumors in a wide variety of tissue types of both epithelial and mesenchymal origin.[10]:107–9 Although viruses in circulation among feral mice can be tumorigenic, under natural conditions the virus does not cause tumors; maternal antibodies have been shown to be critical in protecting neonates.[3][10][11] It has been described as rare in modern laboratory mouse research colonies.[7]
MPyV is also capable of infecting and causing tumors in other rodent species, including guinea pigs, hamsters, and rats, though the diversity of tissue types giving rise to tumors is reduced in these species.[10]:107–9 MPyV does not infect humans and is not associated with human cancers.[12]
Structure
.jpg)
Like other members of the polyomavirus family, MPyV has an unenveloped icosahedral (T=7) viral capsid around 45 nanometers in diameter.[3][13] The capsid contains three proteins; capsid protein VP1 is the primary component and self-assembles into a 360-unit outer capsid layer composed of 72 pentamers. The other two components, VP2 and VP3, have high sequence similarity to each other, with VP3 truncated at the N-terminus relative to VP2. VP2 and VP3 assemble inside the capsid in contact with VP1.[3][13]
VP1 is capable of self-assembly into virus-like particles even in the absence of other viral components.[14] This process requires bound calcium ions and the resulting particles are stabilized by, but do not require, intra-pentamer disulfide bonds.[15]
Genome
MPyV has a closed, circular double-stranded DNA genome of around 5 kilo-base pairs. It contains two transcriptional units located on opposite strands, called the "early region" and "late region" for the stage in the viral life cycle in which they are expressed; each region produces a pre-messenger RNA molecule from which six genes are expressed through alternative splicing. The three genes in the early region express the large, middle, and small tumor antigens (LT, MT, ST) and are sufficient for inducing tumors. The three genes in the late region express the three capsid proteins VP1, VP2, and VP3. Between the early and late regions is a region of noncoding DNA containing the origin of replication and promoter and enhancer elements.[16]:786–7 Expression of a microRNA from a region overlapping one of the LT exons has also been identified and is thought to be involved in downregulating expression of the tumor antigens.[17]
Replication
Cellular entry
Viruses lacking a viral envelope often have complex mechanisms for entry into the host cell. MPyV capsid protein VP1 binds to sialic acids of gangliosides GD1a and GT1b on the cell surface.[1][19] The functions of VP2 and VP3 are less well understood, but at least VP2 has been reported to be exposed upon endocytosis of the viral particle and may be involved in releasing the virus from the endoplasmic reticulum.[20][21] MPyV has been reported to enter cells through both a caveolae-dependent endocytosis mechanism and by an independent mechanism through uncoated vesicles.[21][22]
Unlike many viruses that enter the cell through endocytosis, polyomaviruses penetrate the cell membrane and enter the cytosol from the late endoplasmic reticulum rather than from endosomes, although conformational changes in response to low pH in endolysosomes have been hypothesized as critical steps in the process.[23] MPyV membrane exit is believed to depend on the presence of specific host proteins located in the late ER; for example, the host protein ERp29, a member of the protein disulfide isomerase family, has been shown to disrupt the conformation of VP1.[24] It is not known whether entry into the cytosol is obligatory for MPyV infection or whether the particle could enter the cell nucleus directly from the ER. Even a single viral particle entering the nucleus can be sufficient for infection.[21]
Virion assembly
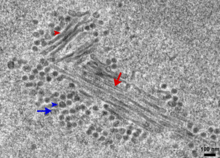
New MPyV virions are assembled in the nucleus in dense local aggregations known as virus factories. Capsid proteins, produced in the cytoplasm of the host cell, enter the nucleus as assembled capsomers consisting of pentameric VP1 associated with VP2 or VP3. Nuclear localization sequences consistent with karyopherin interactions have been identified in capsid protein sequences, facilitating their transit through nuclear pores. Once inside the nucleus they assemble into mature capsids containing a copy of the viral genome, although the exact mechanism of encapsidation is not well understood.[26] Filamentous or tubular structures representing polymerized VP1 have been observed in the nuclei of infected cells as intermediates in the assembly process from which mature virions are produced.[25][27]
Tumorigenesis
MPyV contains three proteins extensively studied for their ability to induce neoplastic transformation (that is, carcinogenesis); these proteins are expressed from the early region of the viral genome and are known as large, middle, and small tumor antigen. Murine polyomavirus and its close relative hamster polyomavirus are historically the only two known viruses whose genomes contain middle tumor antigen, by far the most efficient of the three early proteins at inducing carcinogenesis. In 2015 the genome sequence of a rat polyomavirus was reported to contain middle tumor antigen as well,[28] consistent with expectations that it evolved uniquely in the rodent lineage of the polyomavirus family.[29] Expression of MT from a transgene or introduction in cell culture can be sufficient to induce transformation. Studies using MT have played key roles in understanding host-cell oncogenes and their effects on carcinogenesis, particularly in the study of the Src family of tyrosine kinases.[30] Transgenic mice expressing MT are widely used as models for cancer progression and metastasis, particularly of breast cancer.[31][32][33]
Taxonomy
In the 2015 taxonomic update to the polyomavirus group, the International Committee on Taxonomy of Viruses classified MPyV as the type species of the genus Alphapolyomavirus.[34]
References
- 1 2 Stehle, T; Harrison, SC (15 February 1996). "Crystal structures of murine polyomavirus in complex with straight-chain and branched-chain sialyloligosaccharide receptor fragments.". Structure (London, England : 1993). 4 (2): 183–94. PMID 8805524. doi:10.1016/s0969-2126(96)00021-4.
- ↑ Gross, L (November 1976). "The fortuitous isolation and identification of the polyoma virus.". Cancer Research. 36 (11 Pt 1): 4195–6. PMID 184928.
- 1 2 3 4 Ramqvist, T; Dalianis, T (August 2009). "Murine polyomavirus tumour specific transplantation antigens and viral persistence in relation to the immune response, and tumour development.". Seminars in cancer biology. 19 (4): 236–43. PMID 19505651. doi:10.1016/j.semcancer.2009.02.001.
- ↑ Gross, L. (1953). "A Filterable Agent, Recovered from Ak Leukemic Extracts, Causing Salivary Gland Carcinomas in C3H Mice". Experimental Biology and Medicine. 83 (2): 414–21. PMID 13064287. doi:10.3181/00379727-83-20376.
- ↑ STEWART, SE; EDDY, BE; BORGESE, N (June 1958). "Neoplasms in mice inoculated with a tumor agent carried in tissue culture.". Journal of the National Cancer Institute. 20 (6): 1223–43. PMID 13549981.
- ↑ Eddy, Bernice E.; Stewart, Sarah E. (November 1959). "Characteristics of the SE Polyoma Virus". American Journal of Public Health and the Nations Health. 49 (11): 1486–1492. doi:10.2105/AJPH.49.11.1486.
- 1 2 3 Percy, Dean H.; Barthold, Stephen W. (2013). "Polyoma Virus Infection". Pathology of Laboratory Rodents and Rabbits (3rd ed.). John Wiley & Sons. ISBN 1118704630.
- ↑ Harris, R.J.C. (7 July 1960). "Cancer-inducing Viruses". New Scientist. 8 (190): 21–3.
- ↑ Morgan, Gregory J. (December 2014). "Ludwik Gross, Sarah Stewart, and the 1950s discoveries of Gross murine leukemia virus and polyoma virus". Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences. 48: 200–209. doi:10.1016/j.shpsc.2014.07.013.
- 1 2 3 Fox, James G., ed. (2006). The Mouse in Biomedical Research, Volume 2 Diseases. (2nd ed.). Burlington: Elsevier. ISBN 9780080467719.
- ↑ Carroll, J; Dey, D; Kreisman, L; Velupillai, P; Dahl, J; Telford, S; Bronson, R; Benjamin, T (December 2007). "Receptor-binding and oncogenic properties of polyoma viruses isolated from feral mice.". PLOS Pathogens. 3 (12): e179. PMC 2134959
 . PMID 18085820. doi:10.1371/journal.ppat.0030179.
. PMID 18085820. doi:10.1371/journal.ppat.0030179. - ↑ Cooper, Geoffrey M. (2000). The cell : a molecular approach (2nd ed.). Washington (DC): ASM Press. ISBN 0-87893-106-6.
- 1 2 Ramqvist, T; Dalianis, T (February 2010). "Lessons from immune responses and vaccines against murine polyomavirus infection and polyomavirus-induced tumours potentially useful for studies on human polyomaviruses.". Anticancer research. 30 (2): 279–84. PMID 20332429.
- ↑ Salunke, DM; Caspar, DL; Garcea, RL (12 September 1986). "Self-assembly of purified polyomavirus capsid protein VP1.". Cell. 46 (6): 895–904. PMID 3019556. doi:10.1016/0092-8674(86)90071-1.
- ↑ Schmidt, U; Rudolph, R; Böhm, G (February 2000). "Mechanism of assembly of recombinant murine polyomavirus-like particles.". Journal of Virology. 74 (4): 1658–62. PMC 111640
 . PMID 10644335. doi:10.1128/jvi.74.4.1658-1662.2000.
. PMID 10644335. doi:10.1128/jvi.74.4.1658-1662.2000. - ↑ Lawrence, editor-in-chief, Sir John Kendrew ; executive editor, Eleanor (1994). The encyclopedia of molecular biology. Oxford: Blackwell Science. ISBN 9781444313840.
- ↑ Lagatie, Ole; Tritsmans, Luc; Stuyver, Lieven J (2013). "The miRNA world of polyomaviruses". Virology Journal. 10 (1): 268. PMC 3765807
 . PMID 23984639. doi:10.1186/1743-422X-10-268.
. PMID 23984639. doi:10.1186/1743-422X-10-268. - ↑ Zila, V; Difato, F; Klimova, L; Huerfano, S; Forstova, J (2014). "Involvement of microtubular network and its motors in productive endocytic trafficking of mouse polyomavirus.". PLOS ONE. 9 (5): e96922. PMC 4014599
 . PMID 24810588. doi:10.1371/journal.pone.0096922.
. PMID 24810588. doi:10.1371/journal.pone.0096922. - ↑ Tsai, B; Gilbert, JM; Stehle, T; Lencer, W; Benjamin, TL; Rapoport, TA (1 September 2003). "Gangliosides are receptors for murine polyoma virus and SV40.". The EMBO Journal. 22 (17): 4346–55. PMC 202381
 . PMID 12941687. doi:10.1093/emboj/cdg439.
. PMID 12941687. doi:10.1093/emboj/cdg439. - ↑ Burkert, O; Kreßner, S; Sinn, L; Giese, S; Simon, C; Lilie, H (July 2014). "Biophysical characterization of polyomavirus minor capsid proteins.". Biological chemistry. 395 (7–8): 871–80. PMID 24713574. doi:10.1515/hsz-2014-0114.
- 1 2 3 Tsai, B; Qian, M (2010). "Cellular entry of polyomaviruses.". Current topics in microbiology and immunology. 343: 177–94. PMID 20373089. doi:10.1007/82_2010_38.
- ↑ Gilbert, JM; Benjamin, TL (September 2000). "Early steps of polyomavirus entry into cells.". Journal of Virology. 74 (18): 8582–8. PMC 116371
 . PMID 10954560. doi:10.1128/jvi.74.18.8582-8588.2000.
. PMID 10954560. doi:10.1128/jvi.74.18.8582-8588.2000. - ↑ Qian, M; Cai, D; Verhey, KJ; Tsai, B (June 2009). "A lipid receptor sorts polyomavirus from the endolysosome to the endoplasmic reticulum to cause infection.". PLOS Pathogens. 5 (6): e1000465. PMC 2685006
 . PMID 19503604. doi:10.1371/journal.ppat.1000465.
. PMID 19503604. doi:10.1371/journal.ppat.1000465. - ↑ Magnuson, B; Rainey, EK; Benjamin, T; Baryshev, M; Mkrtchian, S; Tsai, B (28 October 2005). "ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding.". Molecular Cell. 20 (2): 289–300. PMID 16246730. doi:10.1016/j.molcel.2005.08.034.
- 1 2 Erickson, KD; Bouchet-Marquis, C; Heiser, K; Szomolanyi-Tsuda, E; Mishra, R; Lamothe, B; Hoenger, A; Garcea, RL (2012). "Virion assembly factories in the nucleus of polyomavirus-infected cells.". PLOS Pathogens. 8 (4): e1002630. PMC 3320610
 . PMID 22496654. doi:10.1371/journal.ppat.1002630.
. PMID 22496654. doi:10.1371/journal.ppat.1002630. - ↑ Almendral, JM (2013). "Assembly of simple icosahedral viruses.". Sub-cellular biochemistry. 68: 307–28. PMID 23737056. doi:10.1007/978-94-007-6552-8_10.
- ↑ Risco, Cristina; de Castro, Isabel Fernández; Sanz-Sánchez, Laura; Narayan, Kedar; Grandinetti, Giovanna; Subramaniam, Sriram (3 November 2014). "Three-Dimensional Imaging of Viral Infections". Annual Review of Virology. 1 (1): 453–473. doi:10.1146/annurev-virology-031413-085351.
- ↑ Ehlers, B; Richter, D; Matuschka, FR; Ulrich, RG (3 September 2015). "Genome Sequences of a Rat Polyomavirus Related to Murine Polyomavirus, Rattus norvegicus Polyomavirus 1.". Genome announcements. 3 (5): e00997–15. PMC 4559740
 . PMID 26337891. doi:10.1128/genomeA.00997-15.
. PMID 26337891. doi:10.1128/genomeA.00997-15. - ↑ Gottlieb, KA; Villarreal, LP (June 2001). "Natural biology of polyomavirus middle T antigen.". Microbiology and molecular biology reviews : MMBR. 65 (2): 288–318 ; second and third pages, table of contents. PMC 99028
 . PMID 11381103. doi:10.1128/MMBR.65.2.288-318.2001.
. PMID 11381103. doi:10.1128/MMBR.65.2.288-318.2001. - ↑ Fluck, MM; Schaffhausen, BS (September 2009). "Lessons in signaling and tumorigenesis from polyomavirus middle T antigen.". Microbiology and molecular biology reviews : MMBR. 73 (3): 542–63, Table of Contents. PMC 2738132
 . PMID 19721090. doi:10.1128/mmbr.00009-09.
. PMID 19721090. doi:10.1128/mmbr.00009-09. - ↑ Maglione, JE; Moghanaki, D; Young, LJ; Manner, CK; Ellies, LG; Joseph, SO; Nicholson, B; Cardiff, RD; MacLeod, CL (15 November 2001). "Transgenic Polyoma middle-T mice model premalignant mammary disease.". Cancer Research. 61 (22): 8298–305. PMID 11719463.
- ↑ Lin, EY; Jones, JG; Li, P; Zhu, L; Whitney, KD; Muller, WJ; Pollard, JW (November 2003). "Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases.". The American Journal of Pathology. 163 (5): 2113–26. PMC 1892434
 . PMID 14578209. doi:10.1016/s0002-9440(10)63568-7.
. PMID 14578209. doi:10.1016/s0002-9440(10)63568-7. - ↑ Guy, CT; Cardiff, RD; Muller, WJ (March 1992). "Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease.". Molecular and Cellular Biology. 12 (3): 954–61. PMC 369527
 . PMID 1312220. doi:10.1128/mcb.12.3.954.
. PMID 1312220. doi:10.1128/mcb.12.3.954. - ↑ Polyomaviridae Study Group of the International Committee on Taxonomy of, Viruses; Calvignac-Spencer, S; Feltkamp, MC; Daugherty, MD; Moens, U; Ramqvist, T; Johne, R; Ehlers, B (29 February 2016). "A taxonomy update for the family Polyomaviridae.". Archives of Virology. 161: 1739–50. PMID 26923930. doi:10.1007/s00705-016-2794-y.
![]() Media related to MPyV-infected nuclei and MPyV virus factories at Wikimedia Commons
Media related to MPyV-infected nuclei and MPyV virus factories at Wikimedia Commons