Total synthesis of morphine and related alkaloids

Synthesis of morphine-like alkaloids in chemistry describes the total synthesis of the natural morphinan class of alkaloids that includes codeine, morphine, oripavine, and thebaine and the closely related semisynthetic analogs buprenorphine, hydrocodone, isocodeine, naltrexone, naloxone, nalbuphine, and oxycodone.[1][2]
The structure of morphine is not particularly complex, however the electrostatic polarization of adjacent bonded atoms does not alternate uniformly throughout the structure. This "dissonant connectivity" makes bond formation more difficult and therefore significantly complicates any synthetic strategy that is applied to this family of molecules.[2]
The first morphine total synthesis, devised by Marshall D. Gates, Jr. in 1952 remains a widely used example of total synthesis.[3] This synthesis took a total of 31 steps and proceeded in 0.06% overall yield. The dihydrocodeinone synthesis of Kenner C. Rice is one of the most efficient and proceeds in 30% overall yield in 14 steps.[4]
Several other syntheses were reported, notably by the research groups of Evans,[5] Fuchs,[6] Parker,[7] Overman,[8] Mulzer-Trauner,[9] White,[10] Taber,[11] Trost,[12] Fukuyama,[13] Guillou,[14] Stork,[15] and Magnus.[16]
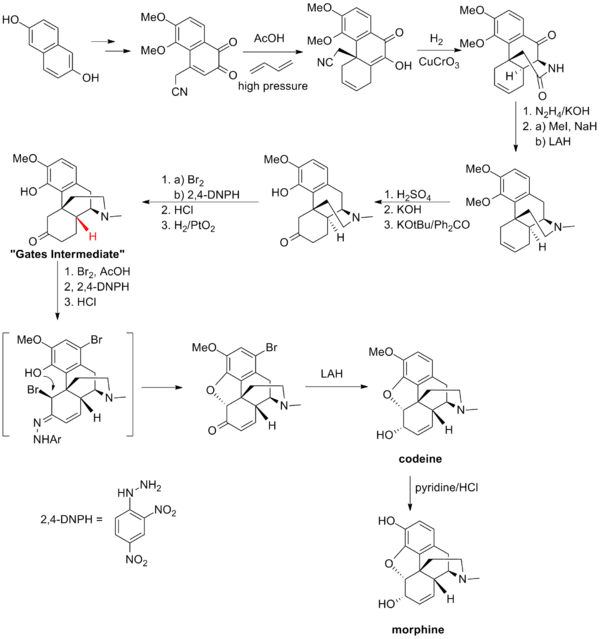
Gates synthesis
Gates' total synthesis of morphine[3] provided a proof of the structure of morphine proposed by Robinson in 1925.[17] This synthesis of morphine features one of the first example of the Diels-Alder reaction in the context of total synthesis.[3]
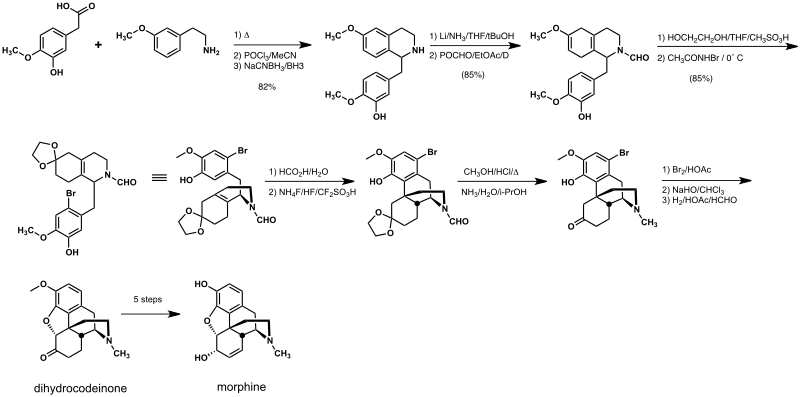
Rice synthesis
The Rice synthesis follows a biomimetic route and is the most efficient reported to date. A key step is the Grewe cyclization that is analogous to the cyclization of reticuline that occurs in morphine biosynthesis.[4]
References
- ↑ Chida N (2011). "Recent advances in the synthesis of morphine and related alkaloids". Top Curr Chem. 299: 1–28. PMID 21630507. doi:10.1007/128_2010_73.
- 1 2 Rinner U, Hudlicky T (2012). "Synthesis of morphine alkaloids and derivatives". Top Curr Chem. 309: 33–66. PMID 21547687. doi:10.1007/128_2011_133.
Morphine's synthesis remains a serious challenge to this day.
- 1 2 3 Gates M, Tschudi G (April 1956). "The Synthesis of Morphine". Journal of the American Chemical Society. 78 (7): 1380–1393. doi:10.1021/ja01588a033.
- 1 2 Rice KC (July 1980). "Synthetic opium alkaloids and derivatives. A short total synthesis of (+/-)-dihydrothebainone, (+/-)-dihydrocodeinone, and (+/-)-nordihydrocodeinone as an approach to a practical synthesis of morphine, codeine, and congeners". The Journal of Organic Chemistry. 45 (15): 3135–3137. doi:10.1021/jo01303a045.
- ↑ Evans DA, Mitch CH (January 1982). "Studies directed towards the total synthesis of morphine alkaloids". Tetrahedron Letters. 23 (3): 285–288. doi:10.1016/S0040-4039(00)86810-0.
- ↑ Toth JE, Hamann PR, Fuchs PL (September 1988). "Studies culminating in the total synthesis of (dl)-morphine". The Journal of Organic Chemistry. 53 (20): 4694–4708. doi:10.1021/jo00255a008.
- ↑ Parker KA, Fokas D (November 1992). "Convergent synthesis of (+/-)-dihydroisocodeine in 11 steps by the tandem radical cyclization strategy. A formal total synthesis of (+/-)-morphine". Journal of the American Chemical Society. 114 (24): 9688–9689. doi:10.1021/ja00050a075.
- ↑ Hong CY, Kado N, Overman LE (November 1993). "Asymmetric synthesis of either enantiomer of opium alkaloids and morphinans. Total synthesis of (−)- and (+)-dihydrocodeinone and (−)- and (+)-morphine". Journal of the American Chemical Society. 115 (23): 11028–11029. doi:10.1021/ja00076a086.
- ↑ Mulzer J, Dürner G, Trauner D (December 1996). "Formal Total Synthesis of(—)-Morphine by Cuprate Conjugate Addition". Angewandte Chemie International Edition in English. 35 (2324): 2830–2832. doi:10.1002/anie.199628301.
- ↑ White JD, Hrnciar P, Stappenbeck F (October 1999). "Asymmetric Total Synthesis of (+)-Codeine via Intramolecular Carbenoid Insertion". The Journal of Organic Chemistry. 64 (21): 7871–7884. doi:10.1021/jo990905z.
- ↑ Taber DF, Neubert TD, Rheingold AL (October 2002). "Synthesis of (−)-Morphine". Journal of the American Chemical Society. 124 (42): 12416–12417. PMID 12381175. doi:10.1021/ja027882h.
- ↑ Trost BM, Tang W (December 2002). "Enantioselective Synthesis of (−)-Codeine and (−)-Morphine". Journal of the American Chemical Society. 124 (49): 14542–14543. PMID 12465957. doi:10.1021/ja0283394.
- ↑ Uchida K, Yokoshima S, Kan T, Fukuyama T (November 2006). "Total Synthesis of (±)-Morphine". Organic Letters. 8 (23): 5311–5313. PMID 17078705. doi:10.1021/ol062112m.
- ↑ Varin M, Barré E, Iorga B, Guillou C (2008). "Diastereoselective Total Synthesis of (±)-Codeine". Chemistry: A European Journal. 14 (22): 6606–6608. doi:10.1002/chem.200800744.
- ↑ Stork G, Yamashita A, Adams J, Schulte GR, Chesworth R, Miyazaki Y, Farmer JJ (2009). "Regiospecific and Stereoselective Syntheses of (±) Morphine, Codeine, and Thebaine via a Highly Stereocontrolled Intramolecular 4 + 2 Cycloaddition Leading to a Phenanthrofuran System". Journal of the American Chemical Society. 131 (32): 11402–11406. PMID 19624126. doi:10.1021/ja9038505.
- ↑ Magnus P, Sane N, Fauber BP, Lynch V (November 2009). "Concise syntheses of (−)-galanthamine and (+/-)-codeine via intramolecular alkylation of a phenol derivative". J. Am. Chem. Soc. 131 (44): 16045–7. PMID 19835379. doi:10.1021/ja9085534.
- ↑ Gulland JM, Robinson R (1925). "Constitution of codeine and thebaine". Memoirs of the Literary and Philosophical Society of Manchester. 69: 79–86.
External links
- Morphine Total Syntheses @ SynArchive.com
- Wilson T (2006). "Synthesis of Morphine Alkaloids" (PDF). Professor Scott E. Denmark Research Group Presentations. University of Illinois at Urbana-Champaign.