Ammonium iron(II) sulfate
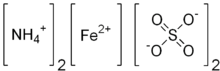 | |
 | |
| | |
| Names | |
|---|---|
| IUPAC name
Ammonium iron(II) sulfate | |
| Other names
Ferrous ammonium sulfate Ammonium iron sulfate Mohr's salt | |
| Identifiers | |
| |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.030.125 |
| EC Number | 233-151-8 |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| (NH4)2Fe(SO4)2·6H2O | |
| Molar mass | 284.05 g mol−1 (anhydrous) 392.13 g mol−1 (hexahydrous) |
| Appearance | Blue-green solid |
| Density | 1.86g/cm3 |
| Melting point | 100 to 110 °C (212 to 230 °F; 373 to 383 K) |
| Boiling point | not applicable |
| 269 g/L (hexahydrate) | |
| Hazards | |
| Main hazards | Irritant (Xi) |
| Safety data sheet | Fisher MSDS |
| R-phrases (outdated) | R36/37/38 |
| S-phrases (outdated) | S24/25 |
| NFPA 704 | |
| Related compounds | |
| Related compounds |
Ammonium iron(III) sulfate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Ammonium iron(II) sulfate, or Mohr's salt, is the inorganic compound with the formula (NH4)2Fe(SO4)2·6H2O. Containing two different cations, Fe2+ and NH4+, it is classified as a double salt of ferrous sulfate and ammonium sulfate. It is a common laboratory reagent. Like the other ferrous sulfate salts, ferrous ammonium sulfate dissolves in water to give the aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry.[1]
Structure
This compound is a member of a group of double sulfates called Schönites or Tutton's salts. Tutton's salts form monoclinic crystals and have formula M2N(SO4)2.6H2O (M = various monocations). With regards to the bonding, crystals consist of octahedra [Fe(OH2)6]2+ centers, which are hydrogen bonded to sulfate and ammonium.[2]

Mohr's salt is named after the German chemist Karl Friedrich Mohr, who made many important advances in the methodology of titration in the 19th century.
Applications
In analytical chemistry, this salt is the preferred source of ferrous ions as the solid has a long shelf life, being resistant to oxidation. This stability extends somewhat to solutions reflecting the effect of pH on the ferrous/ferric redox couple. This oxidation occurs more readily at high pH. The ammonium ions make solutions of Mohr's salt slightly acidic, which slows this oxidation process.[1][3] Sulfuric acid is commonly added to solutions to reduce oxidation to ferric iron.
It is used in the Fricke's dosemeter to measure high doses of gamma rays.[4]
Preparation
Mohr's salt is prepared by dissolving an equimolar mixture of hydrated ferrous sulfate and ammonium sulfate in water containing a little sulfuric acid, and then subjecting the resulting solution to crystallization. Ferrous ammonium sulfate forms light green crystals.
Contaminants
The Analar Standards for Laboratory Chemicals only specify ≥99% purity for the standard Mohr's salt. Before use in titration the salt should be recrystalised, filtered, washed and dried. Common contaminant metal impurities include Mg, Mn, Ni, Pb, and Zn.[5]
References
- 1 2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ Ephraim, Fritz (1926). Inorganic Chemistry. tr P. C. L. Thorne. London: Gurney and Jackson. pp. 484–485.
- ↑ "Ammonium Ferrous Sulphate 100 g (Mohr’s Salt)". 2012. Retrieved 13 June 2013.
- ↑ Hickman, C.; Lorrain, S.; Barthe, J.R.; Portal, G. (1986). "Use of Mohr's Salt for High Level Gamma Dosimetry (Up to 108 Gy)". Radiatiation Protection Dosimetry. Oxford Journals. 17 (1-4): 255–257.
- ↑ Vogel, Arthur I. (1961). A Text-book of Quantitative Inorganic Analysis Including Elementary Instrumental Analysis (3 ed.). Longmans. pp. 281–282.
