Microbial fuel cell
A microbial fuel cell (MFC), or biological fuel cell, is a bio-electrochemical system that drives an electric current by using bacteria and mimicking bacterial interactions found in nature. MFCs can be grouped into two general categories: mediated and unmediated. The first MFCs, demonstrated in the early 20th century, used a mediator: a chemical that transfers electrons from the bacteria in the cell to the anode. Unmediated MFCs emerged in the 1970s; in this type of MFC the bacteria typically have electrochemically active redox proteins such as cytochromes on their outer membrane that can transfer electrons directly to the anode.[1][2] In the 21st century MFCs started to find a commercial use in wastewater treatment.[3]
History
The idea of using microbes to produce electricity was conceived in the early twentieth century. M. C. Potter initiated the subject in 1911.[4] Potter managed to generate electricity from Escherichia coli, but the work received little coverage. In 1931, Branet Cohen created microbial half fuel cells that, when connected in series, were capable of producing over 35 volts with only a current of 2 milliamps.[5]
A study by DelDuca et al. used hydrogen produced by the fermentation of glucose by Clostridium butyricum as the reactant at the anode of a hydrogen and air fuel cell. Though the cell functioned, it was unreliable owing to the unstable nature of hydrogen production by the micro-organisms.[6] This issue was resolved by Suzuki et al. in 1976,[7] who produced a successful MFC design a year later.[8]
In the late 1970s little was understood about how microbial fuel cells functioned. The idea was studied by Robin M. Allen and later by H. Peter Bennetto. People saw the fuel cell as a possible method for the generation of electricity for developing countries. Bennetto's work, starting in the early 1980s, helped build an understanding of how fuel cells operate and he was seen by many as the topic's foremost authority.
In May 2007, the University of Queensland, Australia completed a prototype MFC as a cooperative effort with Foster's Brewing. The prototype, a 10 L design, converted brewery wastewater into carbon dioxide, clean water and electricity. The group had plans to create a pilot-scale model for an upcoming international bio-energy conference.[9]
Definition
A microbial fuel cell (MFC) is a device that converts chemical energy to electrical energy by the action of microorganisms.[10] These electrochemical cells are constructed using either a bioanode and/or a biocathode. Most MFCs contain a membrane to separate the compartments of the anode (where oxidation takes place) and the cathode (where reduction takes place). The electrons produced during oxidation are transferred directly to an electrode or, to a redox mediator species. The electron flux is moved to the cathode. The charge balance of the system is compensated by ionic movement inside the cell, usually across an ionic membrane. Most MFCs use an organic electron donor that is oxidised to produce CO2, protons and electrons. Other electron donors have been reported, such as sulphur compounds or hydrogen.[11] The cathode reaction uses a variety of electron acceptors that includes the reduction of oxygen as the most studied process. However, other electron acceptors have been studied, including metal recovery by reduction,[12] water to hydrogen,[13] nitrate reduction and sulfate reduction.
Applications
Power generation
MFCs are attractive for power generation applications that require only low power, but where replacing batteries may be impractical, such as wireless sensor networks.[14][15][16]
Virtually any organic material could be used to feed the fuel cell, including coupling cells to wastewater treatment plants. MFCs are clean and the best method of energy production. Chemical process wastewater[17][18] and synthetic wastewater[19][20] have been used to produce bioelectricity in dual- and single-chamber mediatorless MFCs (uncoated graphite electrodes).
Higher power production was observed with a biofilm-covered graphite anode.[21][22] Fuel cell emissions are well under regulatory limits.[23] MFCs use energy more efficiently than standard internal combustion engines, which are limited by the Carnot Cycle. In theory, an MFC is capable of energy efficiency far beyond 50%.[24] Rozendal obtained energy conversion to hydrogen 8 times that of conventional hydrogen production technologies.
However; MFCs can also work at a smaller scale. Electrodes in some cases need only be 7 μm thick by 2 cm long.[25] such that an MFC can replace a battery. It provides a renewable form of energy and does not need to be recharged.
MFCs operate well in mild conditions, 20 °C to 40 °C and also at pH of around 7.[26] They lack the stability required for long-term medical applications such as in pacemakers.
Power stations can be based on aquatic plants such as algae. If sited adjacent to an existing power system, the MFC system can share its electricity lines.[27]
Education
Soil-based microbial fuel cells serve as educational tools, as they encompass multiple scientific disciplines (microbiology, geochemistry, electrical engineering, etc.) and can be made using commonly available materials, such as soils and items from the refrigerator. Kits for home science projects and classrooms are available.[28] One example of microbial fuel cells being used in the classroom is in the IBET (Integrated Biology, English, and Technology) curriculum for Thomas Jefferson High School for Science and Technology.
Biosensor
The current generated from a microbial fuel cell is directly proportional to the energy content of wastewater used as the fuel. MFCs can measure the solute concentration of wastewater (i.e., as a biosensor).[29]
Wastewater is commonly assessed for its biochemical oxygen demand (BOD) values. BOD values are determined by incubating samples for 5 days with proper source of microbes, usually activated sludge collected from wastewater plants.
An MFC-type BOD sensor can provide real-time BOD values. Oxygen and nitrate are preferred electron acceptors over the electrode, reducing current generation from an MFC. MFC BOD sensors underestimate BOD values in the presence of these electron acceptors. This can be avoided by inhibiting aerobic and nitrate respiration in the MFC using terminal oxidase inhibitors such as cyanide and azide.[30] Such BOD sensors are commercially available.
The United States Navy is considering microbial fuel cells for environmental sensors. The use of microbial fuel cells to power environmental sensors would be able to provide power for longer periods and enable the collection and retrieval of undersea data without a wired infrastructure. The energy created by these fuel cells is enough to sustain the sensors after an initial startup time.[31] Due to undersea conditions (high salt concentrations, fluctuating temperatures and limited nutrient supply), the Navy may deploy MFCs with a mixture of salt-tolerant microorganisms. A mixture would allow for a more complete utilization of available nutrients. Shewanella oneidensis is their primary candidate, but may include other heat- and cold-tolerant Shewanella spp.[32]
Biorecovery
In 2010, A. ter Heijne et al.[33] constructed a device capable of producing electricity and reducing Cu (II) (ion) to copper metal.
Microbial electrolysis cells have been demonstrated to produce hydrogen.[34]
Wastewater treatment
MFCs are used in water treatment to harvest energy utilizing anaerobic digestion. The process can also reduce pathogens. However, it requires temperatures upwards of 30 degrees C and requires an extra step in order to convert biogas to electricity. Spiral spacers may be used to increase electricity generation by creating a helical flow in the MFC. Scaling MFCs is a challenge because of the power output challenges of a larger surface area.[35]
Types
Mediated
Most microbial cells are electrochemically inactive. Electron transfer from microbial cells to the electrode is facilitated by mediators such as thionine, methyl viologen, methyl blue, humic acid and neutral red.[36][37] Most available mediators are expensive and toxic.
Mediator-free

Mediator-free microbial fuel cells use electrochemically active bacteria to transfer electrons to the electrode (electrons are carried directly from the bacterial respiratory enzyme to the electrode). Among the electrochemically active bacteria are Shewanella putrefaciens,[38] Aeromonas hydrophila[39] and others. Some bacteria are able to transfer their electron production via the pili on their external membrane. Mediator-free MFCs are less well-characterized, such as the strain of bacteria used in the system, type of ion-exchange membrane and system conditions (temperature, pH, etc.)
Mediator-free microbial fuel cells can run on wastewater and derive energy directly from certain plants. This configuration is known as a plant microbial fuel cell. Possible plants include reed sweetgrass, cordgrass, rice, tomatoes, lupines and algae.[40][41][42] Given that the power is derived from living plants (in situ-energy production), this variant can provide ecological advantages.
Microbial electrolysis
One variation of the mediator-less MFC is the microbial electrolysis cell (MEC). While MFCs produce electric current by the bacterial decomposition of organic compounds in water, MECs partially reverse the process to generate hydrogen or methane by applying a voltage to bacteria. This supplements the voltage generated by the microbial decomposition of organics, leading to the electrolysis of water or methane production.[43][44] A complete reversal of the MFC principle is found in microbial electrosynthesis, in which carbon dioxide is reduced by bacteria using an external electric current to form multi-carbon organic compounds.[45]
Soil-based
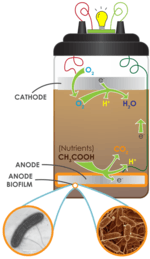
Soil-based microbial fuel cells adhere to the basic MFC principles, whereby soil acts as the nutrient-rich anodic media, the inoculum and the proton exchange membrane (PEM). The anode is placed at a particular depth within the soil, while the cathode rests on top the soil and is exposed to air.
Soils naturally teem with diverse microbes, including electrogenic bacteria needed for MFCs, and are full of complex sugars and other nutrients that have accumulated from plant and animal material decay. Moreover, the aerobic (oxygen consuming) microbes present in the soil act as an oxygen filter, much like the expensive PEM materials used in laboratory MFC systems, which cause the redox potential of the soil to decrease with greater depth. Soil-based MFCs are becoming popular educational tools for science classrooms.[28]
Sediment microbial fuel cells (SMFCs) have been applied for wastewater treatment. Simple SMFCs can generate energy while decontaminating wastewater. Most such SMFCs contain plants to mimic constructed wetlands. By 2015 SMFC tests had reached more than 150 l.[46]
In 2015 researchers announced an SMFC application that extracts energy and charges a battery. Salts dissociate into positively and negatively charged ions in water and move and adhere to the respective negative and positive electrodes, charging the battery and making it possible to remove the salt effecting microbial capacitive desalination. The microbes produce more energy than is required for the desalination process.[47]
Phototrophic biofilm
Phototrophic biofilm MFCs (ner) use a phototrophic biofilm anode containing photosynthetic microorganism such as chlorophytacandyanophyta.They carry out photosynthesis and thus produce organic metabolites and donate electrons.[48]
One study found that PBMFCs display a power density sufficient for practical applications.[49]
The sub-category of phototrophic MFCs that use purely oxygenic photosynthetic material at the anode are sometimes called biological photovoltaic systems.[50]
Nanoporous membrane
The United States Naval Research Laboratory developed nanoporous membrane microbial fuel cells that use a non-PEM to generate passive diffusion within the cell.[51] The membrane is a nonporous polymer filter (nylon, cellulose, or polycarbonate). It offers comparable power densities to Nafion (a well known PEM) with greater durability. Porous membranes allow passive diffusion thereby reducing the necessary power supplied to the MFC in order to keep the PEM active and increasing the total energy output.[52]
MFCs that do not use a membrane can deploy anaerobic bacteria in aerobic environments. However, membrane-less MFCs experience cathode contamination by the indigenous bacteria and the power-supplying microbe. The novel passive diffusion of nanoporous membranes can achieve the benefits of a membrane-less MFC without worry of cathode contamination.
Nanoporous membranes are also eleven times cheaper than Nafion (Nafion-117, $0.22/cm2 vs. polycarbonate, <$0.02/cm2).[53]
Ceramic membrane
PEM membranes can be replaced with ceramic materials. Ceramic membrane costs can be as low as $5.66 /m2. The macroporous structure of ceramic membranes allows good transport of ionic species.[54]
The materials that have been successfully employed in ceramic MFCs are earthenware, alumina, mullite, pyrophyllite and terracotta.[54][55][56]
Generation process
When microorganisms consume a substance such as sugar in aerobic conditions, they produce carbon dioxide and water. However, when oxygen is not present, they produce carbon dioxide, protons and electrons, as described below:[57]
C12H22O11 + 13H2O → 12CO2 + 48H+ + 48e− (Eqt. 1)
Microbial fuel cells use inorganic mediators to tap into the electron transport chain of cells and channel electrons produced. The mediator crosses the outer cell lipid membranes and bacterial outer membrane; then, it begins to liberate electrons from the electron transport chain that normally would be taken up by oxygen or other intermediates.
The now-reduced mediator exits the cell laden with electrons that it transfers to an electrode; this electrode becomes the anode. The release of the electrons recycles the mediator to its original oxidised state, ready to repeat the process. This can happen only under anaerobic conditions; if oxygen is present, it will collect the electrons, as it has greater electronegativity.
In MFC operation, the anode is the terminal electron acceptor recognized by bacteria in the anodic chamber. Therefore, the microbial activity is strongly dependent on the anode's redox potential. A Michaelis-Menten curve was obtained between the anodic potential and the power output of an acetate-driven MFC. A critical anodic potential seems to provide maximum power output.[58]
Potential mediators include natural red, methylene blue, thionine and resorufin.[59]
Organisms capable of producing an electric current are termed exoelectrogens. In order to turn this current into usable electricity, exoelectrogens have to be accommodated in a fuel cell.
The mediator and a micro-organism such as yeast, are mixed together in a solution to which is added a substrate such as glucose. This mixture is placed in a sealed chamber to stop oxygen entering, thus forcing the micro-organism to undertake anaerobic respiration. An electrode is placed in the solution to act as the anode.
In the second chamber of the MFC is another solution and the positively charged cathode. It is the equivalent of the oxygen sink at the end of the electron transport chain, external to the biological cell. The solution is an oxidizing agent that picks up the electrons at the cathode. As with the electron chain in the yeast cell, this could be a variety of molecules such as oxygen, although a more convenient option is a solid oxidizing agent, which requires less volume.
Connecting the two electrodes is a wire (or other electrically conductive path). Completing the circuit and connecting the two chambers is a salt bridge or ion-exchange membrane. This last feature allows the protons produced, as described in Eqt. 1, to pass from the anode chamber to the cathode chamber.
The reduced mediator carries electrons from the cell to the electrode. Here the mediator is oxidized as it deposits the electrons. These then flow across the wire to the second electrode, which acts as an electron sink. From here they pass to an oxidizing material.
Algae Biomass has been observed to give high energy when used as substrates in microbial fuel cell.[60]
See also
- Fermentative hydrogen production
- Biobattery
- Dark fermentation
- Glossary of fuel cell terms
- Photofermentation
- Electrohydrogenesis
- Electromethanogenesis
- Hydrogen technologies
- Hydrogen hypothesis
References
- ↑ Badwal, SPS (2014). "Emerging electrochemical energy conversion and storage technologies". Frontiers in Chemistry. 2: 79. PMC 4174133
 . PMID 25309898. doi:10.3389/fchem.2014.00079.
. PMID 25309898. doi:10.3389/fchem.2014.00079. - ↑ Min, B.; Cheng, S.; Logan, B. E. (2005). "Electricity generation using membrane and salt bridge microbial fuel cells". Water Research. 39 (9): 1675–86. doi:10.1016/j.watres.2005.02.002.
- ↑ "MFC Pilot plant at the Fosters Brewery". Retrieved 2013-03-09.
- ↑ Potter, M.C. Potter (1911). "Electrical effects accompanying the decomposition of organic compounds". Royal Society (Formerly Proceedings of the Royal Society) B. 84: 260–276. doi:10.1098/rspb.1911.0073.
- ↑ Cohen, B (1931). "The Bacterial Culture as an Electrical Half-Cell". Journal of Bacteriology. 21: 18–19.
- ↑ DelDuca, M. G., Friscoe, J. M. and Zurilla, R. W. (1963). Developments in Industrial Microbiology. American Institute of Biological Sciences, 4, pp81–84.
- ↑ Karube, I.; Matasunga, T.; Suzuki, S.; Tsuru, S. (1976). "Continuous hydrogen production by immobilized whole cels of Clostridium butyricum". Biocheimica et Biophysica Acta. 24 (2): 338–343.
- ↑ Karube, Isao; Matsunaga, Tadashi; Tsuru, Shinya; Suzuki, Shuichi (November 1977). "Biochemical cells utilizing immobilized cells of Clostridium butyricum". Biotechnology and Bioengineering. 19 (11): 1727–1733. doi:10.1002/bit.260191112.
- ↑ "Brewing a sustainable energy solution". The University of Queensland Australia. Retrieved 26 August 2014.
- ↑ Allen, R.M.; Bennetto, H.P. (1993). "Microbial fuel cells: Electricity production from carbohydrates". Applied Biochemistry and Biotechnology. 39–40: 27–40. doi:10.1007/bf02918975.
- ↑ Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. (2010). "A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production". Bioresource Technology. 101 (6): 1533–43. PMID 19892549. doi:10.1016/j.biortech.2009.10.017.
- ↑ Lu, Z.; Chang, D.; Ma, J.; Huang, G.; Cai, L.; Zhang, L. (2015). "Behavior of metal ions in bioelectrochemical systems: A review". Journal of Power Sources. 275: 243–260. doi:10.1016/j.jpowsour.2014.10.168.
- ↑ Oh, S.; Logan, B. E. (2005). "Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies". Water Research. 39 (19): 4673–4682. doi:10.1016/j.watres.2005.09.019.
- ↑ Subhas C Mukhopadhyay; Joe-Air Jiang (2013). Wireless Sensor Networks and Ecological Monitoring. Springer link. pp. 151–178. ISBN 978-3-642-36365-8.
- ↑ Chua SL, Wang VB, Cai Z, Sivakumar K, Kjelleberg S, Cao B, Loo SC, Yang L (2014). "A stable synergistic microbial consortium for simultaneous azo dye removal and bioelectricity generation". Bioresource technology. 155: 71–6. PMID 24434696. doi:10.1016/j.biortech.2013.12.078.
- ↑ Chua SL, Wang VB, Cao B, Loo SC, Yang L (2013). "A stable synergistic microbial consortium for simultaneous azo dye removal and bioelectricity generation". PLOS ONE. 8: e63129. PMC 3659106
 . PMID 23700414. doi:10.1371/journal.pone.0063129.
. PMID 23700414. doi:10.1371/journal.pone.0063129. - ↑ Venkata Mohan, S.; Mohanakrishna, G.; Srikanth, S.; Sarma, P. N. (2008). "Harnessing of bioelectricity in microbial fuel cell (MFC) employing aerated cathode through anaerobic treatment of chemical wastewater using selectively enriched hydrogen producing mixed consortia" (PDF). Fuel. 87 (12): 2667–2676. doi:10.1016/j.fuel.2008.03.002.
- ↑ Venkata Mohan, S.; Mohanakrishna, G.; Reddy, B. P.; Saravanan, R.; Sarma, P. N. (2008). "Bioelectricity generation from chemical wastewater treatment in mediatorless (anode) microbial fuel cell (MFC) using selectively enriched hydrogen producing mixed culture under acidophilic microenvironment" (PDF). Biochemical Engineering Journal. 39: 121–130. doi:10.1016/j.bej.2007.08.023.
- ↑ Venkata Mohan, S.; Veer Raghuvulu, S.; Srikanth, S.; Sarma, P.N. (2007). "Bioelectricity production by meditorless microbial fuel cell (MFC) under acidophilic condition using wastewater as substrate: influence of substrate loading rate". Current Sci. 92 (12): 1720–1726.
- ↑ Venkata Mohan, S.; Saravanan, R.; Raghavulu, S. V.; Mohanakrishna, G.; Sarma, P. N. (2008). "Bioelectricity production from wastewater treatment in dual-chamber microbial fuel cell (MFC) using selectively enriched mixed microflora: Effect of catholyte" (PDF). Bioresource Technology. 99 (3): 596–603. PMID 17321135. doi:10.1016/j.biortech.2006.12.026.
- ↑ Venkata Mohan, S.; Veer Raghavulu, S.; Sarma, P. N. (2008). "Biochemical evaluation of bioelectricity production process from anaerobic wastewater treatment in a single-chamber microbial fuel cell (MFC) employing glass wool membrane" (PDF). Biosensors and Bioelectronics. 23 (9): 1326–1332. PMID 18248978. doi:10.1016/j.bios.2007.11.016.
- ↑ Venkata Mohan, S.; Veer Raghavulu, S.; Sarma, P. N. (2008). "Influence of anodic biofilm growth on bioelectricity production in single-chamber mediatorless microbial fuel cell using mixed anaerobic consortia" (PDF). Biosensors and Bioelectronics. 24 (1): 41–47. PMID 18440217. doi:10.1016/j.bios.2008.03.010.
- ↑ Choi Y., Jung S. and Kim S. (2000) Development of Microbial Fuel Cells Using Proteus Vulgaris Bulletin of the Korean Chemical Society, 21 (1), pp44–48
- ↑ Yue & Lowther, 1986
- ↑ Chen, T.; Barton, S.C.; Binyamin, G.; Gao, Z.; Zhang, Y.; Kim, H.-H.; Heller, A. (Sep 2001). "A miniature biofuel cell.". J Am Chem Soc. 123 (35): 8630–1. PMID 11525685. doi:10.1021/ja0163164.
- ↑ Bullen RA, Arnot TC, Lakeman JB, Walsh FC (2006). "Biofuel cells and their development.". Biosensors & Bioelectronics. 21 (11): 2015–45. PMID 16569499. doi:10.1016/j.bios.2006.01.030.
- ↑ Eos magazine, Waterstof uit het riool, June 2008
- 1 2 MudWatt. "MudWatt Science Kit". MudWatt.
- ↑ Kim, BH.; Chang, IS.; Gil, GC.; Park, HS.; Kim, HJ. (April 2003). "Novel BOD (biological oxygen demand) sensor using mediator-less microbial fuel cell.". Biotechnology Letters. 25 (7): 541–545. PMID 12882142. doi:10.1023/A:1022891231369.
- ↑ Chang, I. S.; Moon, H.; Jang, J. K.; Kim, B. H. (2005). "Improvement of a microbial fuel cell performance as a BOD sensor using respiratory inhibitors" (PDF). Biosensors and Bioelectronics. 20 (9): 1856–1859. PMID 15681205. doi:10.1016/j.bios.2004.06.003.
- ↑ Gong, Y., Radachowsky, S. E., Wolf, M., Nielsen, M. E., Girguis, P. R., & Reimers, C. E. (2011). "Benthic Microbial Fuel Cell as Direct Power Source for an Acoustic Modem and Seawater Oxygen/Temperature Sensor System". Environmental Science and Technology. 45 (11): 5047–53. doi:10.1021/es104383q.
- ↑ Biffinger, J.C., Little, B., Pietron, J., Ray, R., Ringeisen, B.R. (2008). "Aerobic Miniature Microbial Fuel Cells". NRL Review: 141–42.
- ↑ Heijne, Annemiek Ter; Liu, Fei; Weijden, Renata van der; Weijma, Jan; Buisman, Cees J.N.; Hamelers, Hubertus V.M. (2010). "Copper Recovery Combined with Electricity Production in a Microbial Fuel Cell". Environmental Science & Technology. 44 (11): 4376–4381. doi:10.1021/es100526g.
- ↑ Heidrick, E S; J. Dolfing; K. Scott; S. R. Edwards; C. Jones; T. P. Curtis (2013). "Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell". Applied Microbiology and Biotechnology. 97 (15): 6979–6989. PMID 23053105. doi:10.1007/s00253-012-4456-7.
- ↑ Zhang, Fei, He, Zhen, Ge, Zheng (2013). "Using Microbial Fuel Cells to Treat Raw Sludge and Primary Effluent for Bioelectricity Generation". Department of Civil Engineering and Mechanics; University of Wisconsin - Milwaukee.
- ↑ Delaney, G. M.; Bennetto, H. P.; Mason, J. R.; Roller, S. D.; Stirling, J. L.; Thurston, C. F. (2008). "Electron-transfer coupling in microbial fuel cells. 2. Performance of fuel cells containing selected microorganism-mediator-substrate combinations". Journal of Chemical Technology and Biotechnology. Biotechnology. 34: 13–27. doi:10.1002/jctb.280340104.
- ↑ Lithgow, A.M., Romero, L., Sanchez, I.C., Souto, F.A., and Vega, C.A. (1986). Interception of electron-transport chain in bacteria with hydrophilic redox mediators. J. Chem. Research, (S):178–179.
- ↑ Kim, B.H.; Kim, H.J.; Hyun, M.S.; Park, D.H. (1999a). "Direct electrode reaction of Fe (III) reducing bacterium, Shewanella putrefacience" (PDF). J Microbiol. Biotechnol. 9: 127–131.
- ↑ Pham, C. A.; Jung, S. J.; Phung, N. T.; Lee, J.; Chang, I. S.; Kim, B. H.; Yi, H.; Chun, J. (2003). "A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell". FEMS Microbiology Letters. 223 (1): 129–134. PMID 12799011. doi:10.1016/S0378-1097(03)00354-9.
- ↑ Mediator-less microbial fuel cell schematic + explanation Archived March 10, 2011, at the Wayback Machine.
- ↑ "Environmental Technology". Wageningen UR.
- ↑ Strik, David; H. V. M. Hamelers; Jan F. H. Snel; Cees J. N. Buisman (July 2008). "Green electricity production with living plants and bacteria in a fuel cell". International Journal of Energy Research. 32 (9): 870–876. doi:10.1002/er.1397.
- ↑ "Advanced Water Management Centre". Advanced Water Management Centre.
- ↑ "DailyTech - Microbial Hydrogen Production Threatens Extinction for the Ethanol Dinosaur".
- ↑ Nevin Kelly P.; Woodard Trevor L.; Franks Ashley E.; et al. (May–June 2010). "Microbial Electrosynthesis: Feeding Microbes Electricity To Convert Carbon Dioxide and Water to Multicarbon Extracellular Organic Compounds". mBio. 1 (2): e00103–10. PMC 2921159
 . PMID 20714445. doi:10.1128/mBio.00103-10.
. PMID 20714445. doi:10.1128/mBio.00103-10. - ↑ Xu, Bojun; Ge, Zheng; He, Zhen (2015). "Sediment microbial fuel cells for wastewater treatment: challenges and opportunities,". Environ. Sci.: Water Res. Technol. The Royal Society of Chemistry,. 1: –,. doi:10.1039/C5EW00020C. Retrieved April 2015. Check date values in:
|access-date=(help) - ↑ Clark, Helen (March 2, 2015). "Cleaning up wastewater from oil and gas operations using a microbe-powered battery". Gizmag. Check date values in:
|access-date=(help); - ↑ Elizabeth, Elmy (2012). "GENERATING ELECTRICITY BY "NATURE’S WAY"". SALT 'B' online magazine. 1.
- ↑ Strik, David; Ruud A. Timmers; Marjolein Helder; Kirsten J.J. Steinbusch; Hubertus V.M. Hamelers; Cees J.N. Buisman (2011). "Microbial solar cells: applying photosynthetic and electrochemically active organisms". Trends in Biotechnology. 29: 41–49. doi:10.1016/j.tibtech.2010.10.001.
- ↑ Bombelli, Paolo; Bradley, Robert W.; Scott, Amanda M.; Philips, Alexander J.; McCormick, Alistair J.; Cruz, Sonia M.; Anderson, Alexander; Yunus, Kamran; Bendall, Derek S.; Cameron, Petra J.; Davies, Julia M.; Smith, Alison G.; Howe, Christopher J.; Fisher, Adrian C. (2011). "Quantitative analysis of the factors limiting solar power transduction by Synechocystis sp. PCC 6803 in biological photovoltaic devices". Energy & Environmental Science. 4 (11): 4690–4698. doi:10.1039/c1ee02531g.
- ↑ "Miniature Microbial Fuel Cells". Technology Transfer Office. Retrieved 30 November 2014.
- ↑ Biffinger, Justin C.; Ray, Ricky; Little, Brenda; Ringeisen, Bradley R. (2007). "Diversifying Biological Fuel Cell Design by Use of Nanoporous Filters". Environmental Science and Technology. 41 (4): 1444–49. doi:10.1021/es061634u.
- ↑ Shabeeba, Anthru (5 Jan 2016). "Seminar 2". Slide Share.
- 1 2 Pasternak, Grzegorz; Greenman, John; Ieropoulos, Ioannis (2016-01-01). "Comprehensive Study on Ceramic Membranes for Low-Cost Microbial Fuel Cells". ChemSusChem. 9 (1): 88–96. ISSN 1864-564X. doi:10.1002/cssc.201501320.
- ↑ Behera, Manaswini; Jana, Partha S.; Ghangrekar, M. M. (2010-02-01). "Performance evaluation of low cost microbial fuel cell fabricated using earthen pot with biotic and abiotic cathode". Bioresource Technology. 101 (4): 1183–1189. doi:10.1016/j.biortech.2009.07.089.
- ↑ Winfield, Jonathan; Greenman, John; Huson, David; Ieropoulos, Ioannis (2013-06-01). "Comparing terracotta and earthenware for multiple functionalities in microbial fuel cells". Bioprocess and Biosystems Engineering. 36 (12): 1913–1921. ISSN 1615-7591. doi:10.1007/s00449-013-0967-6.
- ↑ Bennetto, H. P. (1990). "Electricity Generation by Micro-organisms" (PDF). Biotechnology Education. 1 (4): 163–168.
- ↑ Cheng, Ky; Ho, G; Cord-Ruwisch, R. "Affinity of microbial fuel cell biofilm for the anodic potential.". Environmental Science & Technology. 42 (10): 3828–34. ISSN 0013-936X. doi:10.1021/es8003969.
- ↑ Bennetto, HP.; Stirling, JL.; Tanaka, K.; Vega, CA. (Feb 1983). "Anodic reactions in microbial fuel cells.". Biotechnology and Bioengineering. 25 (2): 559–68. PMID 18548670. doi:10.1002/bit.260250219.
- ↑ Rashid, Naim; Cui, Yu-Feng; Saif Ur Rehman, Muhammad; Han, Jong-In (2013-07-01). "Enhanced electricity generation by using algae biomass and activated sludge in microbial fuel cell". Science of The Total Environment. 456–457: 91–94. doi:10.1016/j.scitotenv.2013.03.067.
- The Biotech/Life Sciences Portal (20 Jan 2006). "Impressive idea – self-sufficient fuel cells". Baden-Württemberg GmbH. Retrieved 2011-02-07.
- Liu H, Cheng S, Logan BE (2005). "Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell". Environ Sci Technol. 32 (2): 658–62. doi:10.1021/es048927c.
- Rabaey, K. & W. Verstraete (2005). "Microbial fuel cells: novel biotechnology for energy generations". Trends Biotechnol. 23 (6): 291–298. PMID 15922081. doi:10.1016/j.tibtech.2005.04.008.
- Yue P.L. and Lowther K. (1986). Enzymatic Oxidation of C1 compounds in a Biochemical Fuel Cell. The Chemical Engineering Journal, 33B, p 69-77
Further reading
- Rabaey, K.; et al. (May 2007). "Microbial ecology meets electrochemistry: electricity-driven and driving communities". Isme J. 1 (1): 9–18. PMID 18043609. doi:10.1038/ismej.2007.4.
- Pant, D.; et al. (March 2010). "A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production". Bioresource Technology. 101 (6): 1533–1543. PMID 19892549. doi:10.1016/j.biortech.2009.10.017.
External links
- DIY MFC Kit
- BioFuel from Microalgae
- Sustainable and efficient biohydrogen production via electrohydrogenesis -Nov 2007
- Microbial Fuel Cell blog A research-type blog on common techniques used in MFC research.
- Microbial Fuel Cells This website is originating from a few of the research groups currently active in the MFC research domain.
- Microbial Fuel Cells from Rhodopherax Ferrireducens An overview from the Science Creative Quarterly.
- Building a Two-Chamber Microbial Fuel Cell
- Discussion group on Microbial Fuel Cells
- Innovation company developing MFC technology