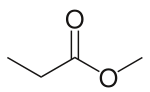
Methyl propionate
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl propanoate | |
| Other names
Methyl propionate Propanoic acid, methyl ester Propionic acid, methyl ester | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.238 |
| PubChem CID |
|
| |
| |
| Properties | |
| C4H8O2 | |
| Molar mass | 88.11 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Density | 0.915 g/mL[1] |
| Melting point | −88 °C (−126 °F; 185 K)[1] |
| Boiling point | 80 °C (176 °F; 353 K)[1] |
| 72 g/L (20 °C)[1] | |
| -55.0·10−6 cm3/mol | |
| Hazards | |
| Flash point | −2 °C (28 °F; 271 K)[1] |
| 465 °C (869 °F; 738 K)[1] | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methyl propionate, also known as methyl propanoate, is a chemical compound with the molecular formula C4H8O2. It is a clear liquid with a fruity, rum-like odor.[2]
Preparation
Methyl propanoate can be prepared by esterification of propionic acid with methanol. Industrially, it is prepared by carboalkoxylation, i.e., the reaction of ethylene with carbon monoxide and methanol in the presence:
- C2H4 + CO + MeOH → MeO2CCH2CH3
The reaction is catalyzed by nickel carbonyl and palladium(0) complexes.[3][4]
Uses
Condensation of Methyl propionate with formaldehyde followed by dehydration yields methyl methacrylate:[4]
- MeO2CCH2CH3 + CH2O → MeO2CCH(CH2OH)CH3
- MeO2CCH(CH2OH)CH3 → MeO2CC(=CH2)CH3
Methyl propionate is used as a solvent for cellulose nitrate and lacquers, and as a raw material for the production of paints, varnishes and other chemicals such as methyl methacrylate.[2][3]
Due to its fruity smell and taste, it is also used in fragrances and flavoring.[2][5]
References
- 1 2 3 4 5 6 7 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- 1 2 3 "Methyl Propionate Hazardous Substance Fact Sheet" (PDF). New Jersey Department of Health and Senior Services.
- 1 2 Ulf-Rainer Samel; Walter Kohler; Armin Otto Gamer; Ullrich Keuser (2000). Propionic Acid and Derivatives. Ullmann's Encyclopedia of Industrial Chemistry. ISBN 9783527306732. doi:10.1002/14356007.a22_223.pub2.(mayth and yafs)
- 1 2 Scott D. Barnicki "Synthetic Organic Chemicals" in Handbook of Industrial Chemistry and Biotechnology edited by James A. Kent, New York : Springer, 2012. 12th ed. ISBN 978-1-4614-4259-2.
- ↑ "Methyl propionate". thegoodscentscompany.com.