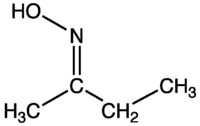
Methylethyl ketone oxime
 | |
| Names | |
|---|---|
| IUPAC name
(2E)-N-Hydroxy-2-butanimine | |
| Other names
MEKO | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.002.270 |
| EC Number | 202-496-6 |
| PubChem CID |
|
| |
| |
| Properties | |
| C4H9NO | |
| Appearance | colourless liquid |
| Density | 0.923 g/cm3 |
| Melting point | −15 °C (5 °F; 258 K) |
| Boiling point | 152 °C (306 °F; 425 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Methylethyl ketone oxime is the organic compound with the formula C2H5C(NOH)CH3. This colourless liquid is the oxime derivative of methyl ethyl ketone. MEKO, as it is called in the paint industry, is used to suppress "skinning" of paints: the formation of a skin on paint before it is used. MEKO functions by binding the drying agents, metal salts that catalyze the oxidative crosslinking of drying oils. Once the paint is applied to a surface, MEKO evaporates, thereby allowing the drying process to proceed. Other antiskinning agents have been used, including phenol-based antioxidants, but these tend to yellow the paint.[1]
References
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.