MAP2K7
Dual specificity mitogen-activated protein kinase kinase 7, also known as MAP kinase kinase 7 or MKK7, is an enzyme that in humans is encoded by the MAP2K7 gene.[5] This protein is a member of the mitogen-activated protein kinase kinase family. The MKK7 protein exists as six different isoforms with three possible N-termini (α, β, and γ isoforms) and two possible C-termini (1 and 2 isoforms).[6]
MKK7 is involved in signal transduction mediating the cell responses to proinflammatory cytokines, and environmental stresses. This kinase specifically activates MAPK8/JNK1 and MAPK9/JNK2, and this kinase itself is phosphorylated and activated by MAP kinase kinase kinases including MAP3K1/MEKK1, MAP3K2/MEKK2, MAP3K3/MEKK5, and MAP4K2/GCK.
MKK7 is ubiquitously expressed in all tissue. However, it displays a higher level of expression in skeletal muscle.[7] Multiple alternatively spliced transcript variants encoding distinct isoforms have been found.[5]
Nomenclature
MAP2K7 is also known as:
- MKK7
- JNK-activated kinase 2
- MAPK/ERK kinase 7 (MEK7)
- Stress-activated protein kinase kinase 4 (SAPK kinase 4, SAPKK4)
- c-Jun N-terminal kinase kinase 2 (JNK kinase 2, JNKK2)
- Stress-activated / extracellular signal-regulated protein kinase kinase 2 (SEK2)
Isoforms
The murine MKK7 protein is encoded by 14 exons which can be alternatively spliced to yield a group of protein kinases. This results in six isoforms with three possible N-termini (α, β, and γ isoforms) and two possible C-termini (1 and 2 isoforms). The molecular mass of the isoforms spans from 38 to 52 kDa, with between 345 and 467 amino acids.[6]
The physiological relevance of the different MKK7 isoforms is still unclear. Evidence shows that the MKK7α, which lacks an NH2-terminal extension, shows a lower basal activity in binding JNK compared to the MKKβ and γ isoforms. The increased basal activity in the β and γ isoforms can be due to the three D-motifs present in the N-terminus of these isoforms.[8]
Structure and function
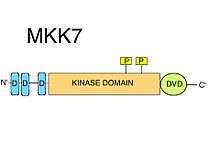
D-motifs
MKK7 has three conserved D-motifs (MAPK-recruiting short linear motifs) on its intinsically disordered N-terminus. D-motifs typically consist of a cluster of positively charged amino acids followed by alternating hydrophobic amino acids.[8] D-motifs are strictly required for the recruitment of MAPKK substrates, such as JNK.[10] The kinase domains of MAPKs contain certain surface features, such as the so-called common docking (CD) region, alongside the docking (D) groove, that specifically recognize their cognate D-motifs.[8] The D-motifs found in MKK7 are highly specific for JNKs, but have a relatively low binding affinity. It was suggested that the motifs of MKK7 can synergize with each other to provide an efficient substrate phosphorylation[11] It has been shown that all three D-motifs are necessary for correct JNK1:MKK7 complex formations, and for the phosphorylation and activation of JNK1 by MKK7.[12]
DVD region
A special extension to the C-terminal kinase domain core, the so-called "Domain for Versatile Docking" (DVD) is a region found in MKK7 as in most known MAP2Ks,.[10] The DVD region is a stable, mostly helical fold of roughly 20 amino acids, that adds onto the back side of the catalytic core of the MAP2K kinase domains.[13] This domain extension is both required for the specific binding to, and activation of MKK7 by respective upstream MAPKKKs. Other mitogen activated protein kinase kinases also require the DVD region (in addition to various other non-canonical elements of their kinase domains, like the "MKK1/2-loop") to be able to discriminate against the various MAPKKK upstream.[14] These special MAPKK:MAPKKK kinase-domain/kinase-domain interactions facilitate the phosphorylation of MKK7.[8] In addition to the activation of MKK7, binding to the DVD region may also affect the MKK7 activation loop in such a way that the Ser and Thr of the S-K-A-K-T motif become accessible for phosphorylation.[8]
Kinase domain
The MKK7 contains one kinase domain. The direct MKK7:MAPKKK interaction (using the DVD region), facilitates the phosphorylation of MKK7 by MAPKKKs on serine and threonine in a S-K-A-K-T motif in the catalytic domain (kinase domain).[9]
Signaling and regulation
MKK7 play an important part in the stress-activated protein kinase/c-Jun N-terminal kinase (SAP/JNK) signaling pathway.[15] In collaboration with another mitogen-activated protein kinase kinase MKK4, MKK7 work as crucial transducers upstream of JNK signaling.[16] Through joint efforts the two MKKs phosphorylate different JNK isoforms. As a result, MKK7 has a great impact on numerous physiological processes such as proliferation and differentiation, as well as pathological processes such as apoptosis and tumorigenesis.[9] MKK7 are activated as a result of cellular stresses.[16] They are activated by a number of MKKKs through phosphorylation at a S-K-A-K-T motif located in the MKK7s kinase domain. The MKKKs relate to MKK7 through its DVD site at the C-terminus and phosphorylate MKK7 at serine and threonine residues.[9] Once activated, MKK4 and MKK7 directly phosphorylate specific tyrosine and threonine residues located in the conserved T-P-Y motif of the activation loop of the JNK protein.[9] Although MKK7 act through dual specificity it tends to phosphorylate threonine on JNK protein, leaving MKK4 to phosphorylate tyrosine.[16] Phosphorylated and activated JNKs activate substrates like transcription factors or pro-apoptotic protein.[9] MKK7 and MKK4 seem to be regulating the expression of each other, thereby affecting the JNK signaling. The mono-phosphorylation of JNK on a threonine residue is adequate for the increase in JNK activity, which argues that MKK7 is an important constituent for JNK activity, while the additional phosphorylation of the tyrosine residue by MKK4 provide for a more favorable activation.[9]
Scaffold proteins
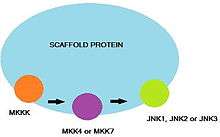
In addition to the direct interactions between JNK, MKK7 and other upstream protein kinases, various scaffold proteins function to ensure specificity between the components of the MAPK signaling cascade.[8][16] Different JNK isoforms, MAPK, and MAPKKs (e.g., MKK7 or MKK4) bind specifically to the scaffold proteins. Several mammalian scaffold proteins have been identified. These include the JNK-interacting protein (JIP) 1 and its closerly-related homolog, JIP2 or the (completely unrelated) JIP3 and JIP4 proteins. Nevertheless, JIP1/2 and JIP3/4 were shown to be capable of direct interaction with each other.[18] Plenty of Src-homology-3 (POSH) has also been shown to be a partner of JIP1/2.[16]
All these JNK pathway regulators assemble transport complexes, tied to kinesin-dependent vesicular transport. In this context, JIP1/2 act as cargo adaptors, binding to a motor protein and a cargo protein simultaneously. In addition to their "normal" cargoes (C-termini of transmembrane proteins), they also transport MAP2K and MAP3K enzymes, namely MKK7, DLK and MLK3. Kinases bound to the JIP1/2 scaffold are generally sequestrated and thought to be inactive.[17] Since the cargo-linkage mechanism of this complex is believed to be phosphporylation-dependent, phosphorylation by JNK kinase can release its own upstream activators from the scaffold, thus driving a strong local positive feedback loop.[17][19]
Interactions
MAP2K7 has been shown to interact with:
Biological relevance
MKK7 is involved in the development of epithelial tissues such as skin and lungs, and also the developing teeth, during early embryogenesis in mice.[8] Experiments also indicate that MKK7 in addition to MKK4 are required for mammalian body plan organization during embryogenesis.[16] MKK7 has also been suggested to function as a Metastase Suppressor Gene (MSG) by promoting tumor dormancy at the metastatic site.[30] In small mammals, stress like pressure overload can cause cardiac hypertrophy and failure if MKK7 is knocked out.[31]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000076984 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000002948 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 "Entrez Gene: MAP2K7 mitogen-activated protein kinase kinase 7".
- 1 2 Tournier C, Whitmarsh AJ, Cavanagh J, Barrett T, Davis RJ (1999). "The MKK7 gene encodes a group of c-Jun NH2-terminal kinase kinases". Molecular and Cellular Biology. 19 (2): 1569–1581. PMC 116085
 . PMID 9891090. doi:10.1128/mcb.19.2.1569.
. PMID 9891090. doi:10.1128/mcb.19.2.1569. - ↑ Foltz IN, Gerl RE, Wieler JS, Luckach M, Salmon RA, Schrader JW (1998). "Human mitogen-activated protein kinase kinase 7 (MKK7) is a highly conserved c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) activated by environmental stresses and physiological stimuli". The Journal of Biological Chemistry. 273 (15): 9344–9351. PMID 9535930. doi:10.1074/jbc.273.15.9344.
- 1 2 3 4 5 6 7 Wang X, Destrument A, Tournier C (2007). "Physiological roles of MKK4 and MKK7: Insights from animal models". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1773 (8): 1349–1357. PMID 17157936. doi:10.1016/j.bbamcr.2006.10.016.
- 1 2 3 4 5 6 7 Haeusgen W, Herdegen T, Waetzig V (2011). "The bottleneck of JNK signaling: Molecular and functional characteristics of MKK4 and MKK7". European Journal of Cell Biology. 90 (6–7): 536–544. PMID 21333379. doi:10.1016/j.ejcb.2010.11.008.
- 1 2 Gantert C, Honerkamp J, Timmer J (1992). "Analyzing the dynamics of hand tremor time series". Biological cybernetics. 66 (6): 479–484. PMID 1586672. doi:10.1007/bf00204112.
- ↑ Ho DT, Bardwell AJ, Abdollahi M, Bardwell L (2003). "A Docking Site in MKK4 Mediates High Affinity Binding to JNK MAPKs and Competes with Similar Docking Sites in JNK Substrates". Journal of Biological Chemistry. 278 (35): 32662–32672. PMC 3017503
 . PMID 12788955. doi:10.1074/jbc.M304229200.
. PMID 12788955. doi:10.1074/jbc.M304229200. - ↑ Ho DT, Bardwell AJ, Grewal S, Iverson C, Bardwell L (2006). "Interacting JNK-docking Sites in MKK7 Promote Binding and Activation of JNK Mitogen-activated Protein Kinases". Journal of Biological Chemistry. 281 (19): 13169–13179. PMC 3017509
 . PMID 16533805. doi:10.1074/jbc.M601010200.
. PMID 16533805. doi:10.1074/jbc.M601010200. - ↑ Raman M, Chen W, Cobb MH (2007). "Differential regulation and properties of MAPKs". Oncogene. 26 (22): 3100–3112. PMID 17496909. doi:10.1038/sj.onc.1210392.
- ↑ Reményi A, Good MC, Lim WA (2006). "Docking interactions in protein kinase and phosphatase networks". Current Opinion in Structural Biology. 16 (6): 676–685. PMID 17079133. doi:10.1016/j.sbi.2006.10.008.
- ↑ Yao Z, Diener K, Wang XS, Zukowski M, Matsumoto G, Zhou G, Mo R, Sasaki T, Nishina H, Hui CC, Tan TH, Woodgett JP, Penninger JM (1997). "Activation of stress-activated protein kinases/c-Jun N-terminal protein kinases (SAPKs/JNKs) by a novel mitogen-activated protein kinase kinase". The Journal of Biological Chemistry. 272 (51): 32378–32383. PMID 9405446. doi:10.1074/jbc.272.51.32378.
- 1 2 3 4 5 6 7 Asaoka Y, Nishina H (2010). "Diverse Physiological Functions of MKK4 and MKK7 during Early Embryogenesis". Journal of Biochemistry. 148 (4): 393–401. PMID 20801953. doi:10.1093/jb/mvq098.
- 1 2 3 Nihalani D, Wong HN, Holzman LB (August 2003). "Recruitment of JNK to JIP1 and JNK-dependent JIP1 phosphorylation regulates JNK module dynamics and activation". J. Biol. Chem. 278 (31): 28694–702. PMID 12756254. doi:10.1074/jbc.M304212200.
- ↑ Hammond JW, Griffin K, Jih GT, Stuckey J, Verhey KJ (May 2008). "Co-operative versus independent transport of different cargoes by Kinesin-1". Traffic. 9 (5): 725–41. PMID 18266909. doi:10.1111/j.1600-0854.2008.00722.x.
- ↑ Nihalani D, Wong H, Verma R, Holzman LB (April 2007). "Src family kinases directly regulate JIP1 module dynamics and activation". Mol. Cell. Biol. 27 (7): 2431–41. PMC 1899903
 . PMID 17242197. doi:10.1128/MCB.01479-06.
. PMID 17242197. doi:10.1128/MCB.01479-06. - ↑ Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, De Smaele E, Tang WJ, D'Adamio L, Franzoso G (2004). "Gadd45β mediates the NF-κB suppression of JNK signalling by targeting MKK7/JNKK2". Nature Cell Biology. 6 (2): 146–153. PMID 14743220. doi:10.1038/ncb1093.
- ↑ Tournier C, Whitmarsh AJ, Cavanagh J, Barrett T, Davis RJ (1997). "Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase". Proceedings of the National Academy of Sciences of the United States of America. 94 (14): 7337–7342. PMC 23822
 . PMID 9207092. doi:10.1073/pnas.94.14.7337.
. PMID 9207092. doi:10.1073/pnas.94.14.7337. - 1 2 Cheng J, Yang J, Xia Y, Karin M, Su B (2000). "Synergistic interaction of MEK kinase 2, c-Jun N-terminal kinase (JNK) kinase 2, and JNK1 results in efficient and specific JNK1 activation". Molecular and Cellular Biology. 20 (7): 2334–2342. PMC 85399
 . PMID 10713157. doi:10.1128/MCB.20.7.2334-2342.2000.
. PMID 10713157. doi:10.1128/MCB.20.7.2334-2342.2000. - ↑ Kelkar N, Gupta S, Dickens M, Davis RJ (2000). "Interaction of a mitogen-activated protein kinase signaling module with the neuronal protein JIP3". Molecular and Cellular Biology. 20 (3): 1030–1043. PMC 85220
 . PMID 10629060. doi:10.1128/MCB.20.3.1030-1043.2000.
. PMID 10629060. doi:10.1128/MCB.20.3.1030-1043.2000. - ↑ Matsuura H, Nishitoh H, Takeda K, Matsuzawa A, Amagasa T, Ito M, Yoshioka K, Ichijo H (2002). "Phosphorylation-dependent scaffolding role of JSAP1/JIP3 in the ASK1-JNK signaling pathway. A new mode of regulation of the MAP kinase cascade". Journal of Biological Chemistry. 277 (43): 40703–40709. PMID 12189133. doi:10.1074/jbc.M202004200.
- 1 2 Yasuda J, Whitmarsh AJ, Cavanagh J, Sharma M, Davis RJ (1999). "The JIP group of mitogen-activated protein kinase scaffold proteins". Molecular and Cellular Biology. 19 (10): 7245–7254. PMC 84717
 . PMID 10490659. doi:10.1128/mcb.19.10.7245.
. PMID 10490659. doi:10.1128/mcb.19.10.7245. - ↑ Merritt SE, Mata M, Nihalani D, Zhu C, Hu X, Holzman LB (1999). "The mixed lineage kinase DLK utilizes MKK7 and not MKK4 as substrate". The Journal of Biological Chemistry. 274 (15): 10195–10202. PMID 10187804. doi:10.1074/jbc.274.15.10195.
- ↑ Negri S, Oberson A, Steinmann M, Sauser C, Nicod P, Waeber G, Schorderet DF, Bonny C (2000). "CDNA Cloning and Mapping of a Novel Islet-Brain/JNK-Interacting Protein". Genomics. 64 (3): 324–330. PMID 10756100. doi:10.1006/geno.2000.6129.
- ↑ Zama T, Aoki R, Kamimoto T, Inoue K, Ikeda Y, Hagiwara M (2002). "Scaffold Role of a Mitogen-activated Protein Kinase Phosphatase, SKRP1, for the JNK Signaling Pathway". Journal of Biological Chemistry. 277 (26): 23919–23926. PMID 11959862. doi:10.1074/jbc.M200838200.
- ↑ Zama T, Aoki R, Kamimoto T, Inoue K, Ikeda Y, Hagiwara M (2002). "A novel dual specificity phosphatase SKRP1 interacts with the MAPK kinase MKK7 and inactivates the JNK MAPK pathway. Implication for the precise regulation of the particular MAPK pathway". Journal of Biological Chemistry. 277 (26): 23909–23918. PMID 11959861. doi:10.1074/jbc.M200837200.
- ↑ Naumov GN, Folkman J, Straume O, Akslen LA (2008). "Tumor-vascular interactions and tumor dormancy". APMIS. 116 (7–8): 569–585. PMID 18834403. doi:10.1111/j.1600-0463.2008.01213.x.
- ↑ Liu, W; Zi, M; Chi, H; Jin, J; Prehar, S; Neyses, L; Cartwright, E. J.; Flavell, R. A.; Davis, R. J.; Wang, X (2011). "Deprivation of MKK7 in cardiomyocytes provokes heart failure in mice when exposed to pressure overload". Journal of Molecular and Cellular Cardiology. 50 (4): 702–11. PMID 21284947. doi:10.1016/j.yjmcc.2011.01.013.
Further reading
- Lu X, Nemoto S, Lin A (1997). "Identification of c-Jun NH2-terminal protein kinase (JNK)-activating kinase 2 as an activator of JNK but not p38.". J. Biol. Chem. 272 (40): 24751–4. PMID 9312068. doi:10.1074/jbc.272.40.24751.
- Wu Z, Wu J, Jacinto E, Karin M (1997). "Molecular cloning and characterization of human JNKK2, a novel Jun NH2-terminal kinase-specific kinase.". Mol. Cell. Biol. 17 (12): 7407–16. PMC 232596
 . PMID 9372971. doi:10.1128/mcb.17.12.7407.
. PMID 9372971. doi:10.1128/mcb.17.12.7407. - Wang Y, Su B, Sah VP, et al. (1998). "Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells.". J. Biol. Chem. 273 (10): 5423–6. PMID 9488659. doi:10.1074/jbc.273.10.5423.
- Yang J, New L, Jiang Y, et al. (1998). "Molecular cloning and characterization of a human protein kinase that specifically activates c-Jun N-terminal kinase.". Gene. 212 (1): 95–102. PMID 9661668. doi:10.1016/S0378-1119(98)00158-9.
- Deacon K, Blank JL (1999). "MEK kinase 3 directly activates MKK6 and MKK7, specific activators of the p38 and c-Jun NH2-terminal kinases.". J. Biol. Chem. 274 (23): 16604–10. PMID 10347227. doi:10.1074/jbc.274.23.16604.
- Karandikar M, Xu S, Cobb MH (2001). "MEKK1 binds raf-1 and the ERK2 cascade components.". J. Biol. Chem. 275 (51): 40120–7. PMID 10969079. doi:10.1074/jbc.M005926200.
- Fleming Y, Armstrong CG, Morrice N, et al. (2001). "Synergistic activation of stress-activated protein kinase 1/c-Jun N-terminal kinase (SAPK1/JNK) isoforms by mitogen-activated protein kinase kinase 4 (MKK4) and MKK7.". Biochem. J. 352 (1): 145–54. PMC 1221441
 . PMID 11062067. doi:10.1042/0264-6021:3520145.
. PMID 11062067. doi:10.1042/0264-6021:3520145. - Vitale G, Bernardi L, Napolitani G, et al. (2001). "Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor.". Biochem. J. 352 (3): 739–45. PMC 1221512
 . PMID 11104681. doi:10.1042/0264-6021:3520739.
. PMID 11104681. doi:10.1042/0264-6021:3520739. - Chayama K, Papst PJ, Garrington TP, et al. (2001). "Role of MEKK2-MEK5 in the regulation of TNF-alpha gene expression and MEKK2-MKK7 in the activation of c-Jun N-terminal kinase in mast cells.". Proc. Natl. Acad. Sci. U.S.A. 98 (8): 4599–604. PMC 31880
 . PMID 11274363. doi:10.1073/pnas.081021898.
. PMID 11274363. doi:10.1073/pnas.081021898. - Acierno JS, Kennedy JC, Falardeau JL, et al. (2001). "A physical and transcript map of the MCOLN1 gene region on human chromosome 19p13.3-p13.2.". Genomics. 73 (2): 203–10. PMID 11318610. doi:10.1006/geno.2001.6526.
- Tournier C, Dong C, Turner TK, et al. (2001). "MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines.". Genes Dev. 15 (11): 1419–26. PMC 312702
 . PMID 11390361. doi:10.1101/gad.888501.
. PMID 11390361. doi:10.1101/gad.888501. - Gross EA, Callow MG, Waldbaum L, et al. (2002). "MRK, a mixed lineage kinase-related molecule that plays a role in gamma-radiation-induced cell cycle arrest.". J. Biol. Chem. 277 (16): 13873–82. PMID 11836244. doi:10.1074/jbc.M111994200.
- Kawaguchi M, Onuchic LF, Huang SK (2002). "Activation of extracellular signal-regulated kinase (ERK)1/2, but not p38 and c-Jun N-terminal kinase, is involved in signaling of a novel cytokine, ML-1.". J. Biol. Chem. 277 (18): 15229–32. PMID 11891214. doi:10.1074/jbc.C100641200.



