Lorlatinib
 | |
| Clinical data | |
|---|---|
| Routes of administration | PO |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| Chemical and physical data | |
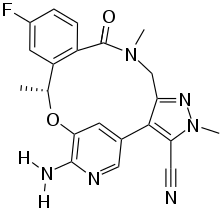
| Formula | C22H20FN5O2 |
| Molar mass | 405.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lorlatinib (PF-6463922) is an experimental anti-neoplastic drug in development by Pfizer. It is a orally-administered small molecule inhibitor of ROS1 and ALK.
In 2015, FDA granted Pfizer orphan drug status for lorlatinib for the treatment of non-small cell lung cancer.[1]
Clinical studies
Several clinical trials are ongoing. A phase II trial comparing avelumab alone and in combination with lorlatinib or crizotinib for non-small cell lung cancer is expected to be complete in late 2017. A phase II trial comparing lorlatinib with crizotinib is expected to be complete in mid-2018.[2] A phase II trial for treatment of ALK-positive or ROS1-positive non-small cell lung cancer with CNA metastases is not expected to be complete until 2023.[3] Preclinical studies are investigating lorlatinib for treatment of neuroblastoma.