List of methylphenidate analogues


This is a list of methylphenidate (MPH or MPD) analogues. Regular methylphenidate can come in several varieties. Including: the racemate, the enantiopure (dextro or levo) of its stereoisomers; erythro or threo (either + or -) among its diastereoisomers, the chiral isomers S,S; S,R/R,S or R,R and, lastly, the isomeric conformers (which are not absolute) of either its anti- or gauche- rotamer. The variant with optimized efficacy is not the usually attested generic or common pharmaceutical brands (e.g. Ritalin, Daytrana etc.) but the (R,R)-dextro-(+)-threo-anti which has a binding profile on par with or better than that of cocaine.[lower-alpha 1] (Note however the measure of fivefold (5×) discrepancy in the entropy of binding at their presumed shared target binding site, which may account for the higher abuse potential of cocaine over methylphenidate despite affinity for associating; i.e the latter dissociates more readily once bound despite efficacy for binding.[lower-alpha 2]) Furthermore, the energy to change between its two rotamers involves the stabilizing of the hydrogen bond between the protonated amine (of an 8.5 pKa) with the ester carbonyl resulting in reduced instances of "gauche—gauche" interactions via its favoring for activity the "anti"-conformer for putative homergic-psychostimulating pharmacokinetic properties, postulating that one inherent conformational isomer ("anti") is necessitated for the activity of the threo diastereoisomer.[lower-alpha 3]
Also of note is that methylphenidate in demethylated form is acidic; a conformation known as ritalinic acid.[2] This gives the potential to yield a conjugate salt[3] form effectively protonated by a salt nearly chemically duplicate/identical to its own structure; creating a "methylphenidate ritalinate".[4]
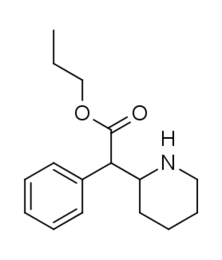
The carboxymethyl (methyl acetate) has sometimes been replaced with similar length ketones to increase duration.[5] For instance, the methoxycarbonyl has had examples of having been replaced with an alkyl group (such as Kozikowski showed with RTI-31 n-propyl residue. cf.[6])
Desoxypipradrol (and thus Pipradrol, including such derivatives as AL-1095, Diphemethoxidine, SCH-5472 & D2PM), and even mefloquine, 2-benzylpiperidine & rimiterol could be considered vaguely related structurally, with the former ones also functionally so, as loosely analogous compounds.
Enpiroline is one further example, loosely related by being based on the 2-benzylpiperidine skeleton, although in its case it is actually heteroaromatic; also of note are etoxadrol and dioxadrol.
Pethidine (Meperidine) is a loose structural analog (being rather between methylphenidate and certain piperidine phenyltropanes) which, though considered functionally an opioid, is also shown to have dopaminergic reuptake qualities.[7]
Common (e.g. well established in literature et attested within grey market depositories) MPH analog compounds
 Racemic methylphenidate
Racemic methylphenidate Enantiopure dextrorotatory
Enantiopure dextrorotatory
("D")methylphenidate
(The stereochemistry on both carbons is dictated by 1 single wedge)
e.g. Focalin Transesterification product of MPH
Transesterification product of MPH
when exposed to ethanol in vivo

 Methylnaphthidate✲
Methylnaphthidate✲
✲The aforementioned specific analogue page has an additional binding values table at its remote internal-link for multiple threo-methylphenidate analogues besides its own. O-2172 The usual methylphenidate piperidine ring has been replaced by a cyclopentane (without the pyrrolidine nitrogen) and a di-chloro at the phenyl ring
O-2172 The usual methylphenidate piperidine ring has been replaced by a cyclopentane (without the pyrrolidine nitrogen) and a di-chloro at the phenyl ring
Staple permutations of ubiquitous simple substitution pattern arrangements of methylphenidate
-(%2B)-ethyl_2-phenyl-2-(2-piperidyl)acetate.png)
-(%2B)-ethyl_2-phenyl-2-(2-piperidyl)acetate.png)
-(%2B)-ethyl_2-phenyl-2-(2-piperidyl)acetate.png)
-(%2B)-ethyl_2-phenyl-2-(2-piperidyl)acetate.png)
Note carboxy has ethyl (i.e. carbethoxy) rather than methyl (i.e. carbmethoxy)
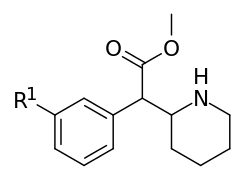
Aryl substitutions
| Compound | S. Singh's alphanumeric assignation (name) |
R1 | R2 | IC50 (nM) (Inhibition of [3H]WIN 35428 binding) |
IC50 (nM) (Inhibition of [3H]DA uptake) |
Selectivity uptake/binding |
|---|---|---|---|---|---|---|
 | ||||||
| (D-threo-methylphenidate) | H, H | 33 | 244 ± 142 (171 ± 10) | 7.4 | ||
| (L-threo-methylphenidate) | 540 | 5100 (1468 ± 112) | 9.4 | |||
| (D/L-threo-methylphenidate) "eudismic ratio" | 6.4 | 20.9 (8.6) | - | |||
| (DL-threo-methylphenidate) | 83.0 ± 7.9 | 224 ± 19 | 2.7 | |||
 | (R-benzoyl-methylecgonine) (cocaine) | (H, H) | 173 ± 13 | 404 ± 26 | 2.3 | |
 | ||||||
| 351a | F | H y d r o g e n i.e. H | 35.0 ± 3.0 | 142 ± 2.0 | 4.1 | |
| 351b | Cl | 20.6 ± 3.4 | 73.8 ± 8.1 | 3.6 | ||
| 351c | Br | 6.9 ± 0.1 | 26.3 ± 5.8 | 3.8 | ||
| 351d | (d) Br | - | 22.5 ± 2.1 | - | ||
| 351e | (l) Br | - | 408 ± 17 | - | ||
| 351d/e "eudismic ratio" | (d/l) Br | - | 18.1 | - | ||
| 351f | I | 14.0 ± 0.1 | 64.5 ± 3.5 | 4.6 | ||
| 351g | OH | 98.0 ± 10 | 340 ± 70 | 3.5 | ||
| 351h | OCH3 | 83 ± 11 | 293 ± 48 | 3.5 | ||
| 351i | (d) OCH3 | - | 205 ± 10 | - | ||
| 351j | (l) OCH3 | - | 3588 ± 310 | - | ||
| 351i/j "eudismic ratio" | (d/l) OCH3 | - | 17.5 | - | ||
| 351k | CH3 | 33.0 ± 1.2 | 126 ± 1 | 3.8 | ||
| 351l | t-Bu | 13500 ± 450 | 9350 ± 950 | 0.7 | ||
| 351m | NH2.HCl | 34.6 ± 4.0 | 115 ± 10 | 3.3 | ||
| 351n | NO2 | 494 ± 33 | 1610 ± 210 | 3.3 | ||
 | ||||||
| 352a | F | 40.5 ± 4.5 | 160 ± 0.00 | 4.0 | ||
| 352b | Cl | 5.1 ± 1.6 | 23.0 ± 3.0 | 4.5 | ||
| 352c | Br | 4.2 ± 0.2 | 12.8 ± 0.20 | 3.1 | ||
| 352d | OH | 321 ± 1.0 | 790 ± 30 | 2.5 | ||
| 352e | OMe | 288 ± 53 | 635 ± 35 | 0.2 | ||
| 352f | Me | 21.4 ± 1.1 | 100 ± 18 | 4.7 | ||
| 352g | NH2.HCl | 265 ± 5 | 578 ± 160 | 2.2 | ||
 | 353a | 2′-F | 1420 ± 120 | 2900 ± 300 | 2.1 | |
| 353b | 2′-Cl | 1950 ± 230 | 2660 ± 140 | 1.4 | ||
| 353c | 2′-Br | 1870 ± 135 | 3410 ± 290 | 1.8 | ||
| 353d | 2′-OH | 23100 ± 50 | 35,800 ± 800 | 1.6 | ||
| 353e | 2′-OCH3 | 101,000 ± 10,000 | 81,000 ± 2000 | 0.8 | ||
 | 354a | Cl, Cl (3′,4′-Cl2) | 5.3 ± 0.7 | 7.0 ± 0.6 | 1.3 | |
| 354b | I | OH | 42 ± 21 | 195 ± 197 | 4.6 | |
| 354c | OMe, OMe (3′,4′-OMe2) | 810 ± 10 | 1760 ± 160 | 2.2 | ||

Both analogues 374 & 375 displayed higher potency than methylphenidate at DAT. In further comparison, 375 (the 2-naphthyl) was additionally two & a half times more potent than 374 (the 1-naphthyl isomer).[lower-alpha 5]

e.g. out from among such examples, the differentiating factor is that the ring formation is left open ("opened" with the excess bond not fused, as above, or omitting one or more bonds on the ring: such as is the case with MABP) in the non-MPH compared analogues.
Aryl exchanged analogues
| Compound | S. Singh's alphanumeric assignation (name) |
Ring | Ki (nM) (Inhibition of [125I]IPT binding) |
Ki (nM) (Inhibition of [3H]DA uptake) |
Selectivity uptake/binding |
|---|---|---|---|---|---|
 | (D-threo-methylphenidate) | benzene | 324 | - | - |
 | (DL-threo-methylphenidate) | 82 ± 77 | 429 ± 88 | 0.7 | |
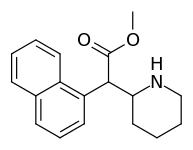 | 374 | 1-naphthalene | 194 ± 15 | 1981 ± 443 | 10.2 |
 | 375 (HDMP-28) | 2-naphthalene | 79.5 | 85.2 ± 25 | 1.0 |
 | 376 | benzyl | >5000 | - | - |

Piperidine nitrogen methylated phenyl-substituted variants
| Compound | S. Singh's alphanumeric assignation (name) |
R | IC50 (nM) (Inhibition of binding at DAT) |
|---|---|---|---|
 | |||
| 373a | H | 500 ± 25 | |
| 373b | 4″-OH | 1220 ± 140 | |
| 373c | 4″-CH3 | 139 ± 13 | |
| 373d | 3″-Cl | 161 ± 18 | |
| 373e | 3″-Me | 108 ± 16 |

Cycloalkane extensions, contractions & modified derivatives
| Compound | S. Singh's alphanumeric assignation (name) |
Cycloalkane ring |
Ki (nM) (Inhibition of binding) |
|---|---|---|---|
 | 380 | 2-pyrrolidine (cyclopentane) | 1336 ± 108 |
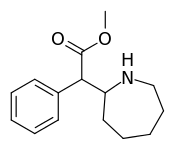 | 381 | 2-azepane (cycloheptane) | 1765 ± 113 |
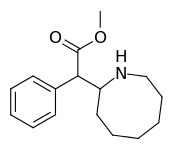 | 382 | 2-azocane (cyclooctane) | 3321 ± 551 |
 | 383 | 4-1,3-oxazinane (cyclohexane) | 6689 ± 1348 |
-2-phenylacetate.svg.png) Methyl 2-(1,2-oxazinan-3-yl)-2-phenylacetate |
-2-phenylacetate.svg.png) Methyl 2-(1,3-oxazinan-2-yl)-2-phenylacetate |
| ☝The two other (in addition to compound 383) potential oxazinane methylphenidate analogues. |
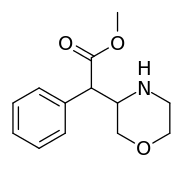 Methyl 2-phenyl-2-(morpholin-3-yl)acetate A.K.A. Methyl 2-morpholin-3-yl-2-phenylacetate | ☜Methylmorphenate methylphenidate analogue.[11] |
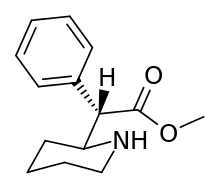
N.B. although the cyclohexane conformation, if considering both the hydrogen on the plain bond and the implicit carbon on the dotted bond are not shown as positioned as would be for the least energy state inherent to what rules apply, internally, to the molecule in and of itself: possibility of movement between putative other ligand sites in suchwise, here regarding what circumstance allows for describing it as "flexed" thus mean it has shown tendency for change in situ depending on its environment and adjacent sites of potential interaction as against its least energy state.
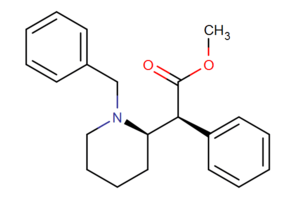
Azido-iodo-N-benzyl analogues
Structures of Azido-iodo-N-benzyl analogues of methylphenidate with affinities.[12]
| Structure | Compound | R1 | R2 | Ki (nM) (Inhibition of [3H]WIN 35428 binding) |
IC50 (nM) (Inhibition of [3H]DA uptake) |
|---|---|---|---|---|---|
| (±)—threo-methylphenidate | H | H | 25 ± 1 | 156 ± 58 | |
| (±)—4-I-methylphenidate | para-iodo | H | 14 ± 3ɑ | 11 ± 2b | |
| (±)—3-I-methylphenidate | meta-iodo | H | 4.5 ± 1ɑ | 14 ± 5b | |
 | |||||
| (±)—p-N3-N-Bn-4-I-methylphenidate | para-iodo | para-N3-N-Benzyl | 363 ± 28ɑ | 2764 ± 196bc | |
| (±)—m-N3-N-Bn-4-I-methylphenidate | para-iodo | meta-N3-N-Benzyl | 2754 ± 169ɑ | 7966 ± 348bc | |
| (±)—o-N3-N-Bn-4-I-methylphenidate | para-iodo | ortho-N3-N-Benzyl | 517 ± 65ɑ | 1232 ± 70bc | |
| (±)—p-N3-N-Bn-3-I-methylphenidate | meta-iodo | para-N3-N-Benzyl | 658 ± 70ɑ | 1828 ± 261bc | |
| (±)—m-N3-N-Bn-3-I-methylphenidate | meta-iodo | meta-N3-N-Benzyl | 2056 ± 73ɑ | 4627 ± 238bc | |
| (±)—o-N3-N-Bn-3-I-methylphenidate | meta-iodo | ortho-N3-N-Benzyl | 1112 ± 163ɑ | 2696 ± 178bc | |
| (±)—N-Bn-methylphenidate | H | N-Benzyl | — | — | |
| (±)—N-Bn-3-chloro-methylphenidate | 3-Cl | N-Benzyl | — | — | |
| (±)—N-Bn-3,4-dichloro-methylphenidate | 3,4-diCl | N-Benzyl | — | — | |
| (±)—p-chloro-N-Bn-methylphenidate | H | para-Cl-N-Benzyl | — | — | |
| (±)—p-methoxy-N-Bn-methylphenidate | H | para-OMe-N-Benzyl | — | — | |
| (±)—m-chloro-N-Bn-methylphenidate | H | meta-Cl-N-Benzyl | — | — | |
| (±)—p-nitro-N-Bn-methylphenidate | H | para-NO2-N-Benzyl | — | — |
- ɑp <0.05 versus Ki of (±)—threo-methylphenidate.
- bp <0.05 versus IC50 of (±)—threo-methylphenidate.
- cp <0.05 versus its corresponding Ki.
Alkyl substituted-carbmethoxy analogues
| Structure | R1 | R2 | R3 | Dopamine transporter Ki (nM) (Inhibition of [I125H]RTI-55 binding) |
DA uptake IC50 (nM) |
Serotonin transporter Ki (nM) (Inhibition of [I125H]RTI-55 binding) |
5HT uptake IC50 (nM) |
Norepinephrine transporter Ki (nM) (Inhibition of [I125H]RTI-55 binding) |
NE uptake IC50 (nM) |
NE/DA selectivity (binding displacement) |
NE/DA selectivity (uptake blocking) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cocaine | — ɑ | — b | — c | 500 ± 65 | 240 ± 15 | 340 ± 40 | 250 ± 40 | 500 ± 90 | 210 ± 30 | 1.0 | 0.88 |
 | |||||||||||
| H | COOCH3 | H | 110 ± 9 | 79 ± 16 | 65,000 ± 4,000 | 5,100 ± 7,000 | 660 ± 50 | 61 ± 14 | 6.0 | 0.77 | |
| 4-chloro | COOCH3 | H | 25 ± 8 2,000 ± 600 | 11 ± 28 2,700 ± 1,000 | 6,000 ± 100 5,900 ± 200 | >9,800 >10 mM | 110 ± 40 >6,100 | 11 ± 3 1,400 ± 400 | 4.4 | 1.0 | |
| 4-chloro | methyl | H | 180 ± 70 >3,900 | 22 ± 7 1,500 ± 700 | 4,900 ± 500 >9,100 | 1,900 ± 300 4,700 ± 800 | 360 ± 140 >6,300 | 35 ± 13 3,200 ± 800 | 2.0 | 1.6 | |
| 4-chloro | ethyl | H | 37 ± 10 1,800 ± 300 | 23 ± 5 2,800 ± 700 | 7,800 ± 800 4,200 ± 400 | 2,400 ± 400 4,100 ± 1,000 | 360 ± 60 >9,200 | 210 ± 30 1,300 ± 400 | 9.7 | 9.1 | |
| 4-chloro | propyl | H | 11 ± 3 380 ± 40 | 7.4 ± 0.4 450 ± 60 | 2,700 ± 600 3,200 ± 1,100 | 2,900 ± 1,100 1,300 ± 7 | 200 ± 80 1,400 ± 400 | 50 ± 15 200 ± 50 | 18.0 | 6.8 | |
| 4-chloro | isopropyl | H | 46 ± 16 900 ± 320 | 32 ± 6 990 ± 280 | 5,300 ± 1,300 >10 mM | 3,300 ± 400 — | 810 ± 170 >10 mM | 51 ± 20 — | 18.0 | 1.6 | |
| 4-chloro | butyl | H | 7.8 ± 1.1 290 ± 70 | 8.2 ± 2.1 170 ± 40 | 4,300 ± 400 4,800 ± 700 | 4,000 ± 400 3,300 ± 600 | 230 ± 30 1,600 ± 300 | 26 ± 7 180 ± 60 | 29.0 | 3.2 | |
| 4-chloro | isobutyl | H | 16 ± 4 170 ± 50 | 8.6 ± 2.9 380 ± 130 | 5,900 ± 900 4,300 ± 500 | 490 ± 80 540 ± 150 | 840 ± 130 4,500 ± 1,500 | 120 ± 40 750 ± 170 | 53.0 | 14.0 | |
| 4-chloro | pentyl | H | 23 ± 7 870 ± 140 | 45 ± 14 650 ± 20 | 2,200 ± 100 3,600 ± 1,000 | 1,500 ± 300 1,700 ± 700 | 160 ± 40 1,500 ± 300 | 49 ± 16 860 ± 330 | 7.0 | 1.1 | |
| 4-chloro | isopentyl | H | 3.6 ± 1.2 510 ± 170 | 14 ± 2 680 ± 120 | 5,000 ± 470 6,700 ± 500 | 7,300 ± 1,400 >8,300 | 830 ± 110 12,000 ± 1,400 | 210 ± 40 3,000 ± 540 | 230.0 | 15.0 | |
| 4-chloro | neopentyl | H | 120 ± 40 600 ± 40 | 60 ± 2 670 ± 260 | 3,900 ± 500 3,500 ± 1,000 | >8,300 1,800 ± 600 | 1,400 ± 400 >5,500 | 520 ± 110 730 ± 250 | 12.0 | 8.7 | |
| 4-chloro | cyclopentylmethyl | H | 9.4 ± 1.5 310 ± 80 | 21 ± 1 180 ± 20 | 2,900 ± 80 3,200 ± 700 | 2,100 ± 900 5,600 ± 1,400 | 1,700 ± 600 2,600 ± 800 | 310 ± 40 730 ± 230 | 180.0 | 15.0 | |
| 4-chloro | cyclohexylmethyl | H | 130 ± 40 260 ± 30 | 230 ± 70 410 ± 60 | 900 ± 400 3,700 ± 500 | 1,000 ± 200 6,400 ± 1,300 | 4,200 ± 200 4,300 ± 200 | 940 ± 140 1,700 ± 600 | 32.0 | 4.1 | |
| 4-chloro | benzyl | H | 440 ± 110 550 ± 60 | 370 ± 90 390 ± 60 | 1,100 ± 200 4,300 ± 800 | 1,100 ± 200 4,700 ± 500 | 2,900 ± 800 4,000 ± 800 | 2,900 ± 600 >8,800 | 6.6 | 7.8 | |
| 4-chloro | phenethyl | H | 24 ± 9 700 ± 90 | 160 ± 20 420 ± 140 | 640 ± 60 1,800 ± 70 | 650 ± 210 210 ± 900d | 1,800 ± 600 2,400 ± 700 | 680 ± 240 610 ± 150 | 75.0 | 4.3 | |
| 4-chloro | phenpropyl | H | 440 ± 150 2,900 ± 900 | 290 ± 90 1,400 ± 400 | 700 ± 200 1,500 ± 200 | 1,600 ± 300 1,200 ± 400 | 490 ± 100 1,500 ± 200 | 600 ± 140 1,700 ± 200 | 1.1 | 2.1 | |
| 4-chloro | 3-pentyl | H | 400 ± 80 >5,700 | 240 ± 60 1,200 ± 90 | 3,900 ± 300 4,800 ± 1,100 | >9,400 >9,600 | 970 ± 290 4,300 ± 200 | 330 ± 80 3,800 ± 30 | 2.4 | 1.4 | |
| 4-chloro | cyclopentyl | H | 36 ± 10 690 ± 140 | 27 ± 8.3 240 ± 30 | 5,700 ± 1,100 4,600 ± 700 | 4,600 ± 800 4,200 ± 900 | 380 ± 120 3,300 ± 800 | 44 ± 18 1,000 ± 300 | 11.0 | 1.6 | |
| 3-chloro | isobutyl | H | 3.7 ± 1.1 140 ± 30 | 2.8 ± 0.4 88 ± 12 | 3,200 ± 400 3,200 ± 400 | 2,100 ± 100 870 ± 230 | 23 ± 6 340 ± 50 | 14 ± 1 73 ± 5 | 6.2 | 5.0 | |
| 3,4-dichloro | COOCH3 | H | 1.4 ± 0.1 90 ± 14 | 23 ± 3 800 ± 110 | 1,600 ± 150 2,500 ± 420 | 540 ± 110 1,100 ± 90 | 14 ± 6 4,200 ± 1,900 | 10 ± 1 190 ± 50 | 10.0 | 0.43 | |
| 3,4-dichloro | propyl | H | 0.97 ± 0.31 43 ± 9 | 4.5 ± 0.4 88 ± 32 | 1,800 ± 500 450 ± 80 | 560 ± 120 180 ± 60 | 3.9 ± 1.4 30 ± 8 | 8.1 ± 3.8 47 ± 22 | 4.0 | 1.8 | |
| 3,4-dichloro | butyl | H | 2.3 ± 0.2 29 ± 5 | 5.7 ± 0.5 67 ± 13 | 1,300 ± 300 1,100 ± 200 | 1,400 ± 300 550 ± 80 | 12 ± 3 31 ± 11 | 27 ± 10 63 ± 27 | 5.2 | 4.7 | |
| 3,4-dichloro | isobutyl | H | 1.0 ± 0.5 31 ± 11 | 5.5 ± 1.3 13 ± 3 | 1,600 ± 100 450 ± 40 | 1,100 ± 300 290 ± 60 | 25 ± 9 120 ± 30 | 9.0 ± 1.2 19 ± 3 | 25.0 | 1.6 | |
| 3,4-dichloro | isobutyl | CH3 | 6.6 ± 0.9 44 ± 12 | 13 ± 4 45 ± 4 | 1,300 ± 200 1,500 ± 300 | 1,400 ± 500 2,400 ± 700 | 190 ± 60 660 ± 130 | 28 ± 3 100 ± 19 | 29.0 | 2.2 | |
| 4-methoxy | isobutyl | H | 52 ± 16 770 ± 220 | 25 ± 9 400 ± 120 | 2,800 ± 600 950 ± 190 | 3,500 ± 500 1,200 ± 300 | 3,100 ± 200 16,000 ± 2,000 | 410 ± 90 1,600 ± 400 | 60.0 | 16.0 | |
| 3-methoxy | isobutyl | H | 22 ± 5 950 ± 190 | 35 ± 12 140 ± 20 | 4,200 ± 400 3,800 ± 600 | 2,700 ± 800 2,600 ± 300 | 3,800 ± 500 12,000 ± 2,300 | 330 ± 40 1,400 ± 90 | 170.0 | 9.4 | |
| 4-isopropyl | isobutyl | H | 3,300 ± 600 >6,500 | 4,000 ± 400 >9,100 | 3,300 ± 600 1,700 ± 500 | 4,700 ± 700 1,700 ± 100 | 2,500 ± 600 3,200 ± 600 | 7,100 ± 1,800 >8,700 | 0.76 | 1.8 | |
| H | COCH3 | H | 370 ± 70 | 190 ± 50 | 7,800 ± 1,200 | >9,700 | 2,700 ± 400 | 220 ± 30 | 7.3 | 1.2 |
- ɑH = Equivalent overlay of structure sharing functional group
- bCO2CH3 (i.e. COOCH3) = Equivalent overlay of structure sharing functional group
- cCH3 = Equivalent overlay of structure sharing functional group
- dpossible typographical error in original source; e.g. 2,100 ± 900 or 900 ± 210
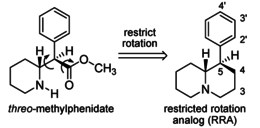
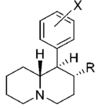
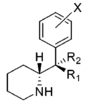
Restricted rotational analogs of methylphenidate (quinolizidines)
Two of the compounds tested, the weakest two @ DAT & second to the final two on the table below, were designed to elucidate the necessity of both constrained rings in the efficacy of the below series of compounds at binding by removing one or the other of the two rings in their entirety. The first of the two retain the original piperidine ring had with methylphenidate but has the constrained B ring that is common to the restricted rotational analogues thereof removed. The one below lacks the piperdine ring native to methylphenidate but keeps the ring that hindered the flexibility of the original MPH conformation. Though their potency at binding is weak in comparison to the series, with the potency shared being approximately equal between the two; the latter compound (the one more nearly resembling the substrate class of dopaminergic releasing agents similar to phenmetrazine) is 8.3-fold more potent @ DA uptake.
| Compoundɑ | R & X substitution(s) | Ki (nM) @ DAT with [33]WIN 35,065-2 |
nH @ DAT with [33]WIN 35,065-2 |
Ki (nM) or % inhibition @ NET with [33]Nisoxetine |
nH @ NET with [33]Nisoxetine |
Ki (nM) or % inhibition @ 5-HTT with [33]Citalopram |
nH @ 5-HTT with [33]Citalopram |
[33]DA uptake IC50 (nM) |
Selectivity [33]Citalopram / [33]WIN 35,065-2 |
Selectivity [33]Nisoxetine / [33]WIN 35,065-2 |
Selectivity [33]Citalopram / [33]Nisoxetine |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cocaine | — | 156 ± 11 | 1.03 ± 0.01 | 1,930 ± 360 | 0.82 ± 0.05 | 306 ± 13 | 1.12 ± 0.15 | 404 ± 26 | 2.0 | 12 | 0.16 |
| Methylphenidate | — | 74.6 ± 7.4 | 0.96 ± 0.08 | 270 ± 23 | 0.76 ± 0.06 | 14 ± 8%f | — | 230 ± 16 | >130 | 3.6 | >47 |
| 3′,4′-dichloro-MPH | — | 4.76 ± 0.62 | 2.07 ± 0.05 | NDh | — | 667 ± 83 | 1.07 ± 0.04 | 7.00 ± 140 | 140 | — | — |
 | |||||||||||
| — | 6,610 ± 440 | 0.91 ± 0.01 | 11%b | — | 3,550 ± 70 | 1.79 ± 0.55 | 8,490 ± 1,800 | 0.54 | >0.76 | <0.7 | |
 | |||||||||||
| H | 76.2 ± 3.4 | 1.05 ± 0.05 | 138 ± 9.0 | 1.12 ± 0.20 | 5,140 ± 670 | 1.29 ± 0.40 | 244 ± 2.5 | 67 | 1.8 | 37 | |
| 3′,4′-diCl | 3.39 ± 0.77 | 1.25 ± 0.29 | 28.4 ± 2.5 | 1.56 ± 0.80 | 121 ± 17 | 1.16 ± 0.31 | 11.0 ± 0.00 | 36 | 8.4 | 4.3 | |
| 2′-Cl | 480 ± 46 | 1.00 ± 0.09 | 2,750; 58%b | 0.96 | 1,840 ± 70 | 1.18 ± 0.06 | 1,260 ± 290 | 3.8 | 5.7 | 0.67 | |
 | |||||||||||
| — | 34.6 ± 7.6 | 0.95 ± 0.18 | 160 ± 18 | 1.28 ± 0.12 | 102 ± 8.2 | 1.01 ± 0.02 | 87.6 ± 0.35 | 3.0 | 4.6 | 0.64 | |
 | |||||||||||
| CH2OH | 2,100 ± 697 | 0.87 ± 0.09 | NDh | — | 16.2 ± 0.05%f | — | 10,400 ± 530 | >4.8 | — | — | |
| CH3 | 7,610 ± 800 | 1.02 ± 0.03 | 8.3%b | — | 11 ± 5%f | — | 7,960 ± 290 | >1.3 | ≫0.66 | — | |
 | |||||||||||
| d R=OCH3, X=H | 570 ± 49 | 0.94 ± 0.10 | 2,040; 64 ± 1.7%f | 0.73 | 14 ± 3%f | — | 1,850 ± 160 | >18 | 3.6 | >4.9 | |
| R=OH, X=H | 6,250 ± 280 | 0.86 ± 0.03 | 23.7 ± 4.1%b | — | 1 ± 1%f | — | 10,700 ± 750 | ≫1.6 | >0.80 | — | |
| R=OH, X=3′,4′-diCl | 35.7 ± 3.2 | 1.00 ± 0.09 | 367 ± 42 | 1.74 ± 0.87 | 2,050 ± 110 | 1.15 ± 0.12 | NDh | 57 | 10 | 5.6 | |
 | |||||||||||
| H | 908 ± 160 | 0.88 ± 0.05 | 4030; 52%b | 1.04 | 5 ± 1%f | — | 12,400 ± 1,500 | ≫11 | 4.4 | ≫2.5 | |
| 3′,4′-diCl | 14.0 ± 1.2 | 1.27 ± 0.20 | 280 ± 76 | 0.68 ± 0.09 | 54 ± 2%f | — | NDh | ~710 | 20 | ~36 | |
 | |||||||||||
| R=OH, X=H | 108 ± 7.0 | 0.89 ± 0.10 | 351 ± 85 | 0.94 ± 0.27 | 12 ± 2%f | — | 680 ± 52 | >93 | 3.3 | >28 | |
| R=OH, X=3′,4′-diCl | 2.46 ± 0.52 | 1.39 ± 0.20 | 27.9 ± 3.5 | 0.70 ± 0.01 | 168 | 1.02 | NDh | 68 | 11 | 6.0 | |
| R=OCH3, X=H | 10.8 ± 0.8 | 0.97 ± 0.07 | 63.7 ± 2.8 | 0.84 ± 0.04 | 2,070; 73 ± 5%f | 0.90 | 61.0 ± 9.3 | 190 | 5.9 | 32 | |
 | |||||||||||
| R1=CH3, R2=H | 178 ± 28 | 1.23 ± 0.09 | 694 ± 65 | 0.88 ± 0.13 | 427 | 1.39 | 368 | 2.4 | 3.9 | 0.62 | |
| R1=H, R2=CH3 | 119 ± 20 | 1.17 ± 0.12 | 76.0 ± 12 | 0.88 ± 0.06 | 243 | 1.17 | 248 | 2.0 | 0.64 | 3.2 | |
 | |||||||||||
| — | 175 ± 8.0 | 1.00 ± 0.04 | 1,520 ± 120 | 0.97 ± 0.06 | 19 ± 4%f | — | NDh | >57 | 8.69 | >6.6 | |
 | |||||||||||
| R=CH2CH3, X=H | 27.6 ± 1.7 | 1.29 ± 0.05 | 441 ± 49 | 1.16 ± 0.19 | 2,390; 80%f | 1.12 | NDh | 87 | 15 | 5.8 | |
| R=CH2CH3, X=3′,4′-diCl | 3.44 ± 0.02 | 1.90 ± 0.05 | 102 ± 19 | 1.27 ± 0.10 | 286 ± 47 | 1.30 ± 0.10 | NDh | 83 | 30 | 2.8 | |
 | |||||||||||
| R=CH2CH3, X=H | 5.51 ± 0.93 | 1.15 ± 0.03 | 60.8 ± 9.6 | 0.75 ± 0.07 | 3,550; 86%f | 0.95 | NDh | 640 | 11 | 58 | |
| R=CH2CH3, X=3′,4′-diCl | 4.12 ± 0.95 | 1.57 ± 0.00 | 98.8 ± 8.7 | 1.07 ± 0.07 | 199 ± 17 | 1.24 ± 0.00 | NDh | 48 | 24 | 2.0 | |
 | |||||||||||
| — | 6,360 ± 1,300 | 1.00 ± 0.04 | 36 ± 10%c | — | 22 ± 7%f | — | 8,800 ± 870 | >1.6 | — | — | |
 | |||||||||||
| i — | 4,560 ± 1,100 | 1.10 ± 0.09 | 534 ± 210c | 0.96 ± 0.08 | 53 ± 6%f | — | 1,060 ± 115 | ~2.2 | 0.12 | ~19 | |
 | |||||||||||
| R1=CH2OH, R2=H, X=H | 406 ± 4 | 1.07 ± 0.08 | NDh | — | 31.0 ± 1.5%f | — | 1,520 ± 15 | >25 | — | — | |
| R1=CH2OCH3, R2=H, X=H | 89.9 ± 9.4 | 0.97 ± 0.04 | NDh | — | 47.8 ± 0.7%f | — | 281 ± 19 | ~110 | — | — | |
| R1=CH2OH, R2=H, X=3′,4′-diCl | 3.91 ± 0.49 | 1.21 ± 0.06 | NDh | — | 276; 94.6%f | 0.89 | 22.5 ± 1.4 | 71 | — | — | |
| R1=H, R2=CO2CH3, X=3′,4′-diCl | 363 ± 20 | 1.17 ± 0.41 | NDh | — | 2,570 ± 580 | 1.00 ± 00.1 | 317 ± 46 | 7.1 | — | — | |
| R1=CO2CH3, R2=H, X=2′-Cl | 1,740 ± 200 | 0.98 ± 0.02 | NDh | — | 22.2 ± 2.5%f | — | 2,660 ± 140 | >5.7 | — | — |
- ɑCompounds tested as hydroclhoride (HCl) salts, unless otherwise noted.
- b% inhibition caused by 5μM
- c% inhibition caused by 10μM, as assayed by SRI
- dTested as free base
- eAssayed by SRI (appropriate correction factor applied.)
- f% inhibition of 10μM compound.
- gValues expressed as x ± SEM of 2—5 replicate tests. (If no SEM shown, value is for an n of 1.)
- hNot determined
- icf. phenmetrazine & derivatives
Various MPH congener affinity values inclusive of norepinephrine & serotonin
Values for dl-threo-methylphenidate derivatives are the mean (s.d.)[16] of 3—6 determinations, or are the mean of duplicate determinations. Values of other compounds are the mean—s.d. for 3—4 determinations where indicated, or are results of single experiments which agree with the literature. All binding experiments were done in triplicate.[17]
| Compound | DA | DA Uptake | NE | 5HT |
|---|---|---|---|---|
| Methylphenidate | 84 ± 33 | 153 ± 92 | 514 ± 74 | >50,000 |
| o-Bromomethylphenidate | 880 ± 316 | — | 20,000 | — |
| m-Bromomethylphenidate | 4 ± 1 | 18 ± 11 | 20 ± 6 | 3,800 |
| p-Bromomethylphenidate | 21 ± 3 | 45 ± 19 | 31 ± 7 | 2,600 |
| p-Hydroxymethylphenidate | 125 | 263 ± 74 | 270 ± 69 | 17,000 |
| p-Methyloxymethylphenidate | 42 ± 24 | 490 ± 270 | 410 | 11,000 |
| p-Nitromethylphenidate | 180 | — | 360 | 5,900 |
| p-Iodomethylphenidate | 26 ± 14 | — | 32 | 1,800ɑ |
| m-Iodo-p-hydroxymethylphenidate | 42 ± 21 | 195 ± 197 | 370 ± 64 | 5,900 |
| N-Methylmethylphenidate | 1,400 | — | 2,800 | 40,000 |
| d-threo-Methylphenidate | 33 | — | 244 ± 142 | >50,000 |
| l-threo-Methylphenidate | 540 | — | 5,100 | >50,000 |
| dl-erythro-o-Bromomethylphenidate | 10,000 | — | 50,000 | — |
| Cocaine | 120 | 313 ± 160 | 2,100 | 190 |
| WIN 35,428 | 13 | — | 530 | 72 |
| Nomifensine | 29 ± 16 | — | 15 ± 2 | 1,300ɑ |
| Mazindol | 9 ± 5 | — | 3 ± 2 | 92 |
| Desipramine | 1,400 | — | 3.5 | 200 |
| Fluoxetine | 3,300 | — | 3,400 | 2.4 |
- ɑDenotes that preparation of membrane and results extrapolated therefrom originated from frozen tissue, which is known to change results when interpreting against fresh tissue experiments.
p-hydroxymethylphenidate displays low brain penetrability, ascribed to its phenolic hydroxyl group undergoing ionization at physiological pH.
Test environment conditioning & control studies
| Compound | 0° (zero degrees) | 0° (zero degrees) Hill slopeɑ |
22° (twenty-two degrees) | 22° (twenty-two degrees) Hill slopeɑ |
36° (thirty-six degrees) | 36° (thirty-six degrees) Hill slopeɑ |
|---|---|---|---|---|---|---|
| Methylphenidate (MPH, MPD) | 51 ± 24 | 0.99 ± 0.11 | 72 ± 29 | 0.90 ± 0.10 | 265 ± 175 | 0.70 ± 0.02 |
| o-bromo-methylphenidate | 1150 ± 83 | 0.97 ± 0.08 | 880 ± 316 | 0.79 ± 0.14 | 954 ± 190 | 0.88 ± 0.08 |
- ɑThe 'Hill' "slope" is a parameter for a biochemical equation named for Archibald Hill; 'degrees' in all cases refer to temperature, measurement of heat & cold, and not to angles. Thus "Hill slope" terminology herein has naught to do with effect of g-force or deviations of a level plane in the context of these values.
See also


External links
piperazine.png)
- Gatley, SJ; Pan, D; Chen, R; Chaturvedi, G; Ding, YS (1996). "Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters". Life Sci. 58: 231–9. PMID 8786705.
- Lapinsky, DJ; Velagaleti, R; Yarravarapu, N; Liu, Y; Huang, Y; Surratt, CK; Lever, JR; Foster, JD; Acharya, R; Vaughan, RA; Deutsch, HM. "Azido-iodo-N-benzyl derivatives of threo-methylphenidate (Ritalin, Concerta): Rational design, synthesis, pharmacological evaluation, and dopamine transporter photoaffinity labeling". Bioorg Med Chem. 19: 504–12. PMC 3023924
 . PMID 21129986. doi:10.1016/j.bmc.2010.11.002.
. PMID 21129986. doi:10.1016/j.bmc.2010.11.002. - "Uses of methylphenidate derivatives" Google patents. Pub. # US 20060183773 A1
- Froimowitz, M; Gu, Y; Dakin, LA; et al. (January 2007). "Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter". J. Med. Chem. 50: 219–32. PMID 17228864. doi:10.1021/jm0608614.
- Davies, HM; Hopper, DW; Hansen, T; Liu, Q; Childers, SR (April 2004). "Synthesis of methylphenidate analogues and their binding affinities at dopamine and serotonin transport sites". Bioorganic. 14: 1799–1802. PMID 15026075. doi:10.1016/j.bmcl.2003.12.097.
- Froimowitz, M; Gu, Y; Dakin, LA; Kelley, CJ; Parrish, D; Deschamps, JR. "Vinylogous amide analogs of methylphenidate". Bioorg Med Chem Lett. 15: 3044–7. PMID 15908207. doi:10.1016/j.bmcl.2005.04.034.
- Schweri, MM; Deutsch, HM; Massey, AT; Holtzman, SG (May 2002). "Biochemical and Behavioral Characterization of Novel Methylphenidate Analogs". Journal of Pharmacology and Experimental Therapeutics. 301: 527–535. PMID 11961053. doi:10.1124/jpet.301.2.527.
- "Methylphenidate analogs with behavioral differences interact differently with arginine residues on the dopamine transporter in rat striatum". Synapse. 57: 175–178. doi:10.1002/syn.20161.
- Quantitative structure-activity relationship studies of threo-methylphenidate analogs.
- Lapinsky, DJ; Yarravarapu, N; Nolan, TL; Surratt, CK; Lever, JR; Tomlinson, M; Vaughan, RA; Deutsch, HM. "Evolution of a Compact Photoprobe for the Dopamine Transporter Based on (±)-threo-Methylphenidate". ACS Med Chem Lett. 3: 378–382. PMC 3469269
 . PMID 23066448. doi:10.1021/ml3000098.
. PMID 23066448. doi:10.1021/ml3000098. - Synthesis and pharmacology of site specific cocaine abuse treatment agents: a new synthetic methodology for methylphenidate analogs based on the Blaise reaction European Journal of Medicinal Chemistry. Howard M Deutsch et al. Volume 36, Issue 4, April 2001, Pages 303–311
References
- 1 2 3 4 5 6 7 8 Singh, Satendra; et al. (2000). "Chemistry, Design, and Structure-Activity Relationship of Cocaine Antagonists" (PDF). Chem. Rev. 100: 925–1024. doi:10.1002/chin.200020238.
- ↑ Marchei, E; Farré, M; Pardo, R; Garcia-Algar, O; Pellegrini, M; Pacifici, R; Pichini, S (2010). "Correlation between methylphenidate and ritalinic acid concentrations in oral fluid and plasma". Clin. Chem. 56: 585–92. PMID 20167695. doi:10.1373/clinchem.2009.138396.
- ↑ Process for the preparation of dexmethylphenidate hydrochloride Google patents; Publication #US 20040180928 A1
- ↑ Resolution of ritalinic acid salt Google patents; Publication #US6441178 B2
- 1 2 3 Froimowitz, Mark; Gu, Yonghong; Dakin, Les A.; Nagafuji, Pamela M.; Kelley, Charles J.; Parrish, Damon; Deschamps, Jeffrey R.; Janowsky, Aaron (2007). "Slow-Onset, Long-Duration, Alkyl Analogues of Methylphenidate with Enhanced Selectivity for the Dopamine Transporter". Journal of Medicinal Chemistry. 50 (2): 219–232. ISSN 0022-2623. PMID 17228864. doi:10.1021/jm0608614.
- ↑ Froimowitz, M.; Gu, Y.; Dakin, L.; Nagafuji, P.; Kelley, C.; Parrish, D.; Deschamps, J.; Janowsky, A. (2007). "Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter". Journal of Medicinal Chemistry. 50 (2): 219–232. PMID 17228864. doi:10.1021/jm0608614.
- ↑ Izenwasser, S; Newman, AH; Cox, BM; Katz, JL (February 1996). "The cocaine-like behavioral effects of meperidine are mediated by activity at the dopamine transporter". Eur. J. Pharmacol. 297 (1-2): 9–17. PMID 8851160. doi:10.1016/0014-2999(95)00696-6.
- ↑ Markowitz, J. S.; Zhu, H. J.; Patrick, K. S. (2013). "Isopropylphenidate: An Ester Homolog of Methylphenidate with Sustained and Selective Dopaminergic Activity and Reduced Drug Interaction Liability". Journal of Child and Adolescent Psychopharmacology. 23 (10): 648–54. PMID 24261661. doi:10.1089/cap.2013.0074.
- 1 2 Kim, Deog-Il; Deutsch, Howard M.; Ye, Xiaocong; Schweri, Margaret M. (2007). "Synthesis and Pharmacology of Site-Specific Cocaine Abuse Treatment Agents: Restricted Rotation Analogues of Methylphenidate". Journal of Medicinal Chemistry. 50 (11): 2718–2731. ISSN 0022-2623. PMID 17489581. doi:10.1021/jm061354p.
- ↑ The Reinforcing Efficacy of Psychostimulants in Rhesus Monkeys: The Role of Pharmacokinetics and Pharmacodynamics 0022-3565/03/3071-356–366 The Journal Of Pharmacology And Experimental Therapeutics. Vol. 307, No. 1
- ↑ U.S. National Library of Medicine, PubChem Compound Summary for CID 85054562
- 1 2 Lapinsky, David J.; Velagaleti, Ranganadh; Yarravarapu, Nageswari; Liu, Yi; Huang, Yurong; Surratt, Christopher K.; Lever, John R.; Foster, James D.; Acharya, Rejwi; Vaughan, Roxanne A.; Deutsch, Howard M. (2011). "Azido-iodo-N-benzyl derivatives of threo-methylphenidate (Ritalin, Concerta): Rational design, synthesis, pharmacological evaluation, and dopamine transporter photoaffinity labeling". Bioorganic & Medicinal Chemistry. 19 (1): 504–512. ISSN 0968-0896. PMC 3023924
 . PMID 21129986. doi:10.1016/j.bmc.2010.11.002.
. PMID 21129986. doi:10.1016/j.bmc.2010.11.002. - ↑ European Bioinformatics Institute (EMBL-EBI) ChEMBL database (ChEMBL1254008 page)
- ↑ European Bioinformatics Institute (EMBL-EBI) ChEMBL database (ChEMBL1255099 page)
- ↑ Jones, DC; Miller, GW (2008). "The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction". Biochemical Pharmacology. 76 (5): 569–81. PMID 18555207. doi:10.1016/j.bcp.2008.05.010.
- ↑ Jaykaran. ""Mean ± SEM" or "Mean (SD)"?". Indian J Pharmacol. 42: 329. PMC 2959222
 . PMID 21206631. doi:10.4103/0253-7613.70402.
. PMID 21206631. doi:10.4103/0253-7613.70402. - ↑ Gatley, SJ; Pan, D; Chen, R; Chaturvedi, G; Ding, YS (1996). "Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters". Life Sci. 58 (12): 231–9. PMID 8786705.
- ↑ Prinz, Heino (Mar 2010). "Hill coefficients, dose–response curves and allosteric mechanisms". J Chem Biol. 3 (1): 37–44. PMC 2816740
 . PMID 19779939. doi:10.1007/s12154-009-0029-3.
. PMID 19779939. doi:10.1007/s12154-009-0029-3. - ↑ Endrenyi, Laszlo; Fajszi, Csaba; Kwong, F. H. F. (1975). "Evaluation of Hill Slopes and Hill Coefficients when the Saturation Binding or Velocity is not Known". European Journal of Biochemistry. 51 (2): 317–28. doi:10.1111/j.1432-1033.1975.tb03931.x. External link in
|title=(help) - ↑ "Computational tools for fitting the Hill equation to dose–response curves". Journal of Pharmacological and Toxicological Methods. 71: 68–76. doi:10.1016/j.vascn.2014.08.006.
- ↑ [1] ←Page #1,005 (81st page of article) §VI. Final ¶.
- ↑ [1] ←Page #1,006 (82nd page of article) 2nd column, end of first ¶.
- ↑ [1] ←Page #1,005 (81st page of article) Final § (§VI.) & page #1,006 (82nd page of article) left (1st) column, first ¶ and figure 51.
- ↑ [1] ←Page #1,010 (86th page of article) Table 47, Page #1,007 (83rd page of article) Figure 52
- ↑ [1] ←Page #1,010 (86th page of article) 2nd ¶, lines 2, 3 & 5.
- ↑ [1] ←Page #1,010 (86th page of article) Table 49, Page #1,007 (83rd page of article) Figure 54
- ↑ [1] ←Page #1,010 (86th page of article) Table 48, Page #1,007 (83rd page of article) Figure 53
- ↑ [1] ←Page #1,011 (87th page of article) Table 50, Page #1,007 (83rd page of article) Figure 55
| Wikimedia Commons has media related to Methylphenidate. |

