Lithium cobalt oxide
 | |
 | |
| Names | |
|---|---|
| IUPAC name
lithium cobalt(III) oxide | |
| Other names
lithium cobaltite | |
| Identifiers | |
| ECHA InfoCard | 100.032.135 |
| PubChem CID |
|
| Properties | |
| LiCoO 2 | |
| Molar mass | 97.87 g mol−1 |
| Hazards | |
| Main hazards | harmful |
| R-phrases (outdated) | R42/43 |
| S-phrases (outdated) | S36 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Lithium cobalt oxide (LiCoO
2) is a chemical compound commonly used in the positive electrodes of lithium-ion batteries. The structure of LiCoO
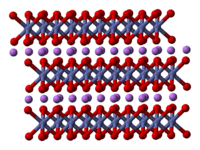
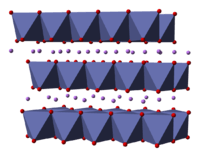
2 has been studied with numerous techniques including x-ray diffraction, electron microscopy, neutron powder diffraction, and EXAFS:[2] it consists of layers of lithium that lie between slabs of octahedra formed by cobalt and oxygen atoms.[3] The space group is [4] in Hermann-Mauguin notation, signifying a rhombus-like unit cell with threefold improper rotational symmetry and a mirror plane. More simply, however, both lithium and cobalt are octahedrally coordinated by oxygen. These octahedra are edge-sharing, and tilted relative to the layered structure. The threefold rotational axis (which is normal to the layers) is termed improper because the triangles of oxygen (being on opposite sides of each octahedron) are anti-aligned.
Batteries produced with LiCoO
2 cathodes have very stable capacities, but have lower capacities and power than cathodes based on nickel-cobalt-aluminum (NCA) oxides. Issues with thermal stability are better for LiCoO
2 cathodes than other nickel-rich chemistries although not significantly. This makes LiCoO
2 batteries susceptible to thermal runaway in cases of abuse such as high temperature operation (>130 °C) or overcharging. At elevated temperatures, LiCoO
2 decomposition generates oxygen, which then reacts with the organic electrolyte of the cell. This is a safety concern due to the magnitude of this highly exothermic reaction, which can spread to adjacent cells or ignite nearby combustible material.[5] In general, this is seen for many lithium ion battery cathodes. LiCoO
2 is widely used as the cathode active material in consumer electronic devices. The performance of LiCoO
2 in batteries is related with the particle size of the material and different sizes from nanometer to micrometer sized particles are researched and produced.[6][7]
The compound's usefulness as an intercalation electrode was discovered in 1980[8] by John B. Goodenough's research group at Oxford.
References
- ↑ 442704 - Lithium cobalt(III) oxide (2012-09-14). "Sigma-Aldrich product page". Sigmaaldrich.com. Retrieved 2013-01-21.
- ↑ I. Nakai; K. Takahashi; Y. Shiraishi; T. Nakagome; F. Izumi; Y. Ishii; F. Nishikawa; T. Konishi (1997). "X-ray absorption fine structure and neutron diffraction analyses of de-intercalation behavior in the LiCoO2 and LiNiO2 systems". Journal of Power Sources. 68 (2): 536–539. doi:10.1016/S0378-7753(97)02598-6.
- ↑ Yang Shao-Horn; Laurence Croguennec; Claude Delmas; E. Chris Nelson; Michael A. O'Keefe (July 2003). "Atomic resolution of lithium ions in LiCoO
2". Nature Materials. 2 (7): 464–467. PMID 12806387. doi:10.1038/nmat922. - ↑ H. J. Orman & P. J. Wiseman (January 1984). "Cobalt(III) lithium oxide, CoLiO
2: structure refinement by powder neutron diffraction". Acta Crystallographica Section C. 40 (1): 12–14. doi:10.1107/S0108270184002833. - ↑ Doughty, Daniel; Pesaran, Ahmad. "Vehicle Battery Safety Roadmap Guidance" (PDF). National Renewable Energy Laboratory. Retrieved 19 January 2013.
- ↑ Tang, W.; Liu, L. L.; Tian, S.; Li, L.; Yue, Y. B.; Wu, Y. P.; Guan, S. Y.; Zhu, K. (2010-11-01). "Nano-LiCoO2 as cathode material of large capacity and high rate capability for aqueous rechargeable lithium batteries". Electrochemistry Communications. 12 (11): 1524–1526. doi:10.1016/j.elecom.2010.08.024.
- ↑ Qi, Zhaoxiang (August 2016). "High-Performance LiCoO2 Sub-Micrometer Materials from Scalable Microparticle Template Processing". ChemistrySelect. 1: 3992–3999.
- ↑ K. Mizushima; P.C. Jones; P.J. Wiseman; J.B. Goodenough (1980). "Li
xCoO
2 (0<x<l): A NEW CATHODE MATERIAL FOR BATTERIES OF HIGH ENERGY DENSITY". Materials Research Bulletin. 15: 783–789. doi:10.1016/0025-5408(80)90012-4.
External links
- Imaging the Structure of Lithium Cobalt Oxide at Atomic Level from the Lawrence Berkeley National Laboratory