Lattice constant
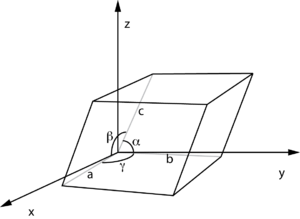
The lattice constant, or lattice parameter, refers to the physical dimension of unit cells in a crystal lattice. Lattices in three dimensions generally have three lattice constants, referred to as a, b, and c. However, in the special case of cubic crystal structures, all of the constants are equal and we only refer to a. Similarly, in hexagonal crystal structures, the a and b constants are equal, and we only refer to the a and c constants. A group of lattice constants could be referred to as lattice parameters. However, the full set of lattice parameters consist of the three lattice constants and the three angles between them.
For example, the lattice constant for diamond is a = 3.57 Å at 300 K. The structure is equilateral although its actual shape cannot be determined from only the lattice constant. Furthermore, in real applications, typically the average lattice constant is given. Near the crystal's surface, lattice constant is affected by the surface reconstruction that results in a deviation from its mean value. This deviation is especially important in nanocrystals since surface-to-nanocrystal core ratio is large.[2] As lattice constants have the dimension of length, their SI unit is the meter. Lattice constants are typically on the order of several ångströms (i.e. tenths of a nanometer). Lattice constants can be determined using techniques such as X-ray diffraction or with an atomic force microscope. Lattice constant of a crystal can be used as a natural length standard of nanometer range.[3][4][5]
In epitaxial growth, the lattice constant is a measure of the structural compatibility between different materials. Lattice constant matching is important for the growth of thin layers of materials on other materials; when the constants differ, strains are introduced into the layer, which prevents epitaxial growth of thicker layers without defects.
Volume
The volume of the unit cell can be calculated from the lattice constant lengths and angles. If the unit cell sides are represented as vectors, then the volume is the dot product of one vector with the cross product of the other two vectors. The volume is represented by the letter V. For the general unit cell
For monoclinic lattices with α = 90°, γ = 90°, this simplifies to
For orthorhombic, tetragonal and cubic lattices with β = 90° as well, then[6]
Lattice matching
Matching of lattice structures between two different semiconductor materials allows a region of band gap change to be formed in a material without introducing a change in crystal structure. This allows construction of advanced light-emitting diodes and diode lasers.
For example, gallium arsenide, aluminium gallium arsenide, and aluminium arsenide have almost equal lattice constants, making it possible to grow almost arbitrarily thick layers of one on the other one.
Lattice grading
Typically, films of different materials grown on the previous film or substrate are chosen to match the lattice constant of the prior layer to minimize film stress.
An alternative method is to grade the lattice constant from one value to another by a controlled altering of the alloy ratio during film growth. The beginning of the grading layer will have a ratio to match the underlying lattice and the alloy at the end of the layer growth will match the desired final lattice for the following layer to be deposited.
The rate of change in the alloy must be determined by weighing the penalty of layer strain, and hence defect density, against the cost of the time in the epitaxy tool.
For example, indium gallium phosphide layers with a band gap above 1.9 eV can be grown on gallium arsenide wafers with index grading.
List of lattice constants
| Material | Lattice constant (Å) | Crystal structure | Ref. |
|---|---|---|---|
| C (diamond) | 3.567 | Diamond (FCC) | [7] |
| C (graphite) | a = 2.461 c = 6.708 | Hexagonal | |
| Si | 5.431 | Diamond (FCC) | [8] |
| Ge | 5.658 | Diamond (FCC) | [8] |
| AlAs | 5.6605 | Zinc blende (FCC) | [8] |
| AlP | 5.4510 | Zinc blende (FCC) | [8] |
| AlSb | 6.1355 | Zinc blende (FCC) | [8] |
| GaP | 5.4505 | Zinc blende (FCC) | [8] |
| GaAs | 5.653 | Zinc blende (FCC) | [8] |
| GaSb | 6.0959 | Zinc blende (FCC) | [8] |
| InP | 5.869 | Zinc blende (FCC) | [8] |
| InAs | 6.0583 | Zinc blende (FCC) | [8] |
| InSb | 6.479 | Zinc blende (FCC) | [8] |
| MgO | 4.212 | Halite (FCC) | [9] |
| SiC | a = 3.086 c = 10.053 | Wurtzite | [8] |
| CdS | 5.8320 | Zinc blende (FCC) | [7] |
| CdSe | 6.050 | Zinc blende (FCC) | [7] |
| CdTe | 6.482 | Zinc blende (FCC) | [7] |
| ZnO | a = 3.25 c = 5.2 | Wurtzite (HCP) | [10] |
| ZnO | 4.580 | Halite (FCC) | [7] |
| ZnS | 5.420 | Zinc blende (FCC) | [7] |
| PbS | 5.9362 | Halite (FCC) | [7] |
| PbTe | 6.4620 | Halite (FCC) | [7] |
| BN | 3.6150 | Zinc blende (FCC) | [7] |
| BP | 4.5380 | Zinc blende (FCC) | [7] |
| CdS | a = 4.160 c = 6.756 | Wurtzite | [7] |
| ZnS | a = 3.82 c = 6.26 | Wurtzite | [7] |
| AlN | a = 3.112 c = 4.982 | Wurtzite | [8] |
| GaN | a = 3.189 c = 5.185 | Wurtzite | [8] |
| InN | a = 3.533 c = 5.693 | Wurtzite | [8] |
| LiF | 4.03 | Halite | |
| LiCl | 5.14 | Halite | |
| LiBr | 5.50 | Halite | |
| LiI | 6.01 | Halite | |
| NaF | 4.63 | Halite | |
| NaCl | 5.64 | Halite | |
| NaBr | 5.97 | Halite | |
| NaI | 6.47 | Halite | |
| KF | 5.34 | Halite | |
| KCl | 6.29 | Halite | |
| KBr | 6.60 | Halite | |
| KI | 7.07 | Halite | |
| RbF | 5.65 | Halite | |
| RbCl | 6.59 | Halite | |
| RbBr | 6.89 | Halite | |
| RbI | 7.35 | Halite | |
| CsF | 6.02 | Halite | |
| CsCl | 4.123 | Caesium chloride | |
| CsI | 4.567 | Caesium chloride | |
| Al | 4.046 | FCC | [11] |
| Fe | 2.856 | BCC | [11] |
| Ni | 3.499 | FCC | [11] |
| Cu | 3.597 | FCC | [11] |
| Mo | 3.142 | BCC | [11] |
| Pd | 3.859 | FCC | [11] |
| Ag | 4.079 | FCC | [11] |
| W | 3.155 | BCC | [11] |
| Pt | 3.912 | FCC | [11] |
| Au | 4.065 | FCC | [11] |
| Pb | 4.920 | FCC | [11] |
| TiN | 4.249 | Halite | |
| ZrN | 4.577 | Halite | |
| HfN | 4.392 | Halite | |
| VN | 4.136 | Halite | |
| CrN | 4.149 | Halite | |
| NbN | 4.392 | Halite | |
| TiC | 4.328 | Halite | [12] |
| ZrC0.97 | 4.698 | Halite | [12] |
| HfC0.99 | 4.640 | Halite | [12] |
| VC0.97 | 4.166 | Halite | [12] |
| NC0.99 | 4.470 | Halite | [12] |
| TaC0.99 | 4.456 | Halite | [12] |
| Cr3C2 | a = 11.47 b = 5.545 c = 2.830 | Orthorombic | [12] |
| WC | a = 2.906 c = 2.837 | Hexagonal | [12] |
| ScN | 4.52 | Halite | [13] |
| LiNbO3 | a = 5.1483 c = 13.8631 | Hexagonal | [14] |
| KTaO3 | 3.9885 | Cubic perovskite | [14] |
| BaTiO3 | a = 3.994 c = 4.034 | Tetragonal perovskite | [14] |
| SrTiO3 | 3.98805 | Cubic perovskite | [14] |
| CaTiO3 | a = 5.381 b = 5.443 c = 7.645 | Orthorhombic perovskite | [14] |
| PbTiO3 | a = 3.904 c = 4.152 | Tetragonal perovskite | [14] |
| EuTiO3 | 7.810 | Cubic perovskite | [14] |
| SrVO3 | 3.838 | Cubic perovskite | [14] |
| CaVO3 | 3.767 | Cubic perovskite | [14] |
| BaMnO3 | a = 5.673 c = 4.71 | Hexagonal | [14] |
| CaMnO3 | a = 5.27 b = 5.275 c = 7.464 | Orthorhombic perovskite | [14] |
| SrRuO3 | a = 5.53 b = 5.57 c = 7.85 | Orthorhombic perovskite | [14] |
| YAlO3 | a = 5.179 b = 5.329 c = 7.37 | Orthorhombic perovskite | [14] |
References
- ↑ "Unit cell definition using parallelepiped with lengths a, b, c and angles between the sides given by α, β, γ". Archived from the original on 4 October 2008.
- ↑ Abdulsattar, Mudar A. (2011). "Ab initio large unit cell calculations of the electronic structure of diamond nanocrystals". Solid State Sci. 13: 843. doi:10.1016/j.solidstatesciences.2011.03.009.
- ↑ Lapshin, R. V. (1998). "Automatic lateral calibration of tunneling microscope scanners". Review of Scientific Instruments. 69 (9): 3268–3276. Bibcode:1998RScI...69.3268L. ISSN 0034-6748. doi:10.1063/1.1149091.
- ↑ Lapshin, R. V. (2015). "Drift-insensitive distributed calibration of probe microscope scanner in nanometer range: Approach description". Applied Surface Science. 359: 629–636. Bibcode:2015ApSS..359..629L. ISSN 0169-4332. arXiv:1501.05545
 . doi:10.1016/j.apsusc.2015.10.108.
. doi:10.1016/j.apsusc.2015.10.108. - ↑ Lapshin, R. V. (2016). "Drift-insensitive distributed calibration of probe microscope scanner in nanometer range: Virtual mode". Applied Surface Science. 378: 530–539. ISSN 0169-4332. doi:10.1016/j.apsusc.2016.03.201.
- ↑ Dept. of Crystallography & Struc. Biol. CSIC (4 June 2015). "4. Direct and reciprocal lattices". Retrieved 9 June 2015.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Lattice Constants". Argon National Labs (Advanced Photon Source). Retrieved 19 October 2014.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 "Semiconductor NSM". Retrieved 19 October 2014.
- ↑ "Substrates". Spi Supplies. Retrieved 17 May 2017.
- ↑ Hadis Morkoç and Ümit Özgur (2009). Zinc Oxide: Fundamentals, Materials and Device Technology. Weinheim: WILEY-VCH Verlag GmbH & Co.
- 1 2 3 4 5 6 7 8 9 10 11 Davey, Wheeler (1925). "Precision Measurements of the Lattice Constants of Twelve Common Metals". Physical Review. 25: 753–761. Bibcode:1925PhRv...25..753D. doi:10.1103/PhysRev.25.753.
- 1 2 3 4 5 6 7 8 Toth, L.E. (1967). Transition Metal Carbides and Nitrides. New York: Academic Press.
- ↑ Saha, B. (2010). "Electronic structure, phonons, and thermal properties of ScN, ZrN, and HfN: A first-principles study". Journal of Applied Physics. 107: 033715. Bibcode:2010JAP...107c3715S. doi:10.1063/1.3291117.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Goodenough, J. B.; Longo, M. "3.1.7 Data: Crystallographic properties of compounds with perovskite or perovskite-related structure, Table 2 Part 1". SpringerMaterials - The Landolt-Börnstein Database.