Lactide
 | |
| Names | |
|---|---|
| Other names
Dilactid, (R,R)-3,6-Dimethyl-1,4-dioxan-2,5-dion, (S,S)-3,6-Dimethyl-1,4-dioxan-2,5-dion, (meso)-3,6-Dimethyl-1,4-dioxan-2,5-dion, (R,R)-2,5-Dimethyl-3,6-dioxo-1,4-dioxan, (S,S)-2,5-Dimethyl-3,6-dioxo-1,4-dioxan, (meso)-2,5-Dimethyl-3,6-dioxo-1,4-dioxan | |
| Identifiers | |
| |
| ECHA InfoCard | 100.002.245 |
| Properties | |
| C6H8O4 | |
| Molar mass | 144.13 g·mol−1 |
| Melting point | 95 to 97 °C (203 to 207 °F; 368 to 370 K) [(S,S)-Lactide and (R,R)-Lactide][2] |
| Hydrolyses to lactic acid[2] | |
| Solubility | soluble in chloroform, methanol slightly soluble in benzene |
| Hazards | |
| R-phrases (outdated) | R36/37/38 |
| S-phrases (outdated) | S26 S37/39 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Lactide is the cyclic di-ester of lactic acid, i.e., 2-hydroxypropionic acid. Lactic acid cannot form a lactone as other hydroxy acids do because the hydroxy group is too close to the carboxylic group. Instead, lactic acid first forms a dimer, which is similar to a 5-hydroxyacid. The dimer contains a hydroxy group at a convenient distance from the carboxylic group for the formation of a lactone. Indeed, the dimer readily forms a six-membered cyclic diester known as lactide. Lactides may be prepared by heating lactic acid in the presence of an acid catalyst.
In general, a lactide is the cyclic diester, i.e., the di-lactone of two molecules of any 2-hydroxycarboxylic acid.
Stereoisomers
Lactic acid is chiral; two enantiomeric forms, (R)-lactic acid and (S)-lactic acid, may exist. Thus, lactide formed from two equivalents of lactic acid consists of two stereocenters. Three different stereoisomers of lactide are known:
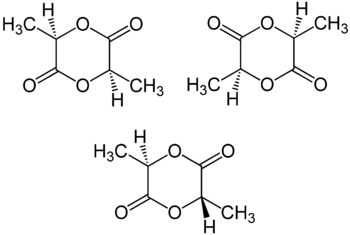
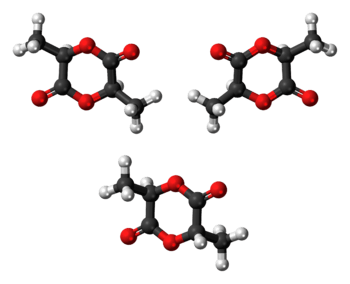
Polymerization
Lactide can be polymerized to polylactic acid (polylactide) using suitable catalysts, with either syndiotactic or a heterotactic stereocontrol, to give materials with many useful properties:[3]
References
- ↑ Sigma Aldrich product page for lactide Retrieved 8th of July 2015
- 1 2 3 Römpp Online Chemielexikon Version 3.3 aufgerufen am 25. März 2009
- ↑ R. Auras; L.-T. Lim; S. E. M. Selke; H. Tsuji (2010). Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications. Wiley. ISBN 978-0-470-29366-9.
