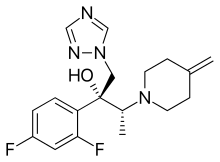
Efinaconazole
 | |
| Clinical data | |
|---|---|
| Trade names | Jublia, Clenafin |
| License data |
|
| Routes of administration | Topical (solution) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unknown (oral) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C18H22F2N4O |
| Molar mass | 348.39 g/mol |
| 3D model (JSmol) | |
| |
| |
Efinaconazole (trade names Jublia and Clenafin) is a triazole antifungal. It is approved for use in Canada and the USA, JAPAN as a 10% topical solution for the treatment of onychomycosis (fungal infection of the nail).[1][2] Efinaconazole acts as a 14α-demethylase inhibitor.[3][4]
In two clinical trials 17.8% and 15.2% of patients using efinaconazole were cured, compared to 3.3% and 5.5% of patients using a placebo.[4]
Efinaconazole is not especially effective, but it is currently the best topical treatment available, with cure rates two or three times better than the next best topical treatment, ciclopirox. It is considered a reasonable option for patients with mild cases, or patients who can not take oral treatment.[5]
In 2014, the U.S. Food and Drug Administration (FDA) approved the New Drug Application (NDA) for Jublia, the "first topical triazole approved for the treatment of onychomycosis of the toenails."[6] According to Valeant Pharmaceuticals International Inc CEO J. Michael Pearson they acquired Jublia through their purchase of Dow Pharmaceutical Sciences in 2008.[6]
References
- ↑ Patel T, Dhillon S (Nov 2013). "Efinaconazole: first global approval". Drugs. 73 (17): 1977–1983. PMID 24249649. doi:10.1007/s40265-013-0152-x.
- ↑ Tschen EH, Bucko AD, Oizumi N, Kawabata H, Olin JT, Pillai R (Feb 2013). "Efinaconazole solution in the treatment of toenail onychomycosis: a phase 2, multicenter, randomized, double-blind study". J Drugs Dermatol. 12 (2): 186–192. PMID 23377392.
- ↑ Tatsumi Y, Nagashima M, Shibanushi T, et al. (May 2013). "Mechanism of action of efinaconazole, a novel triazole antifungal agent". Antimicrob Agents Chemother. 57 (5): 2405–2509. doi:10.1128/aac.02063-12.
- 1 2 "Drugs at FDA: JUBLIA" (PDF). Retrieved 26 June 2014.
- ↑ "A Closer Look At A New Topical Option For Onychomycosis". Retrieved 21 May 2015.
- 1 2 "Valeant Pharmaceuticals Announces FDA Approval Of Jublia® for the Treatment of Onychomycosis". Valeant Pharmaceuticals. Laval, Quebec. 9 June 2014. Retrieved 1 November 2015.