Ullmann condensation
| Ullmann condensation | |
|---|---|
| Named after | Fritz Ullmann |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | ullmann-reaction |
| RSC ontology ID | RXNO:0000081 |
The Ullmann condensation or Ullmann ether synthesis is a variation of the Ullmann reaction, in which a phenol is coupled to an aryl halide to create a diaryl ether in the presence of a copper compound, named after Fritz Ullmann.[1] The corresponding aniline or aryl amide reaction is sometimes called Goldberg reaction, named after Irma Goldberg.[2]
An example is the synthesis of p-nitrophenyl phenyl ether [3]

An active copper powder that is required for this reaction can be prepared by the reduction of copper sulfate by zinc metal in hot water causing the precipitation of elemental copper.
The reaction often requires high-boiling polar solvents such as N-methylpyrrolidone, nitrobenzene or dimethylformamide and high temperatures (often in excess of 210 °C) with stoichiometric amounts of copper. The aryl halide is activated by electron-withdrawing groups or carries a carboxylic acid group in the aromatic ortho position.[4] The research field was revitalized with the introduction of catalytic copper reactions[5] in 2001–2003 using up to 0.1 equivalent copper iodide, base and a diamine ligand.
Goldberg reaction
An example of a Goldberg reaction is the synthesis of fenamic acid, an intermediate of acridone via the Goldberg reaction:[6]
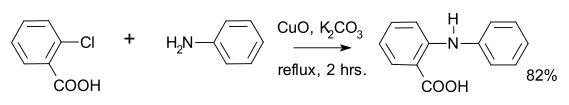
An Ullmann-type aromatic amination reaction between an aryl iodide and an aryl amine as coupling partners has been published.[7] The catalyst used is formed from copper(I) iodide and phenanthroline. As this reaction proceeds well with an electron-rich aryl iodide it is a valuable alternative to the Buchwald–Hartwig amination reaction, which gives best yields with electron-poor aryl halides.
The scope is extended to amides [8][9][10] for example in the synthesis of this Camps cyclization precursor:[11]

Hurtley reaction
The nucleophile can also be carbon as in a carbanion in the Hurtley reaction.[12]
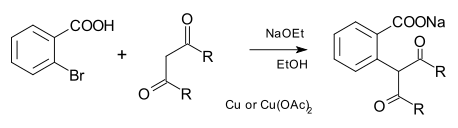
In the original scope the arene was 2-bromobenzoic acid, the carbon nucleophile a malonic ester and other dicarbonyl compounds and the base sodium ethoxide.
References
- ↑ Fritz Ullmann, Paul Sponagel (1905). "Ueber die Phenylirung von Phenolen". Berichte der deutschen chemischen Gesellschaft. 38 (2): 2211–2212. doi:10.1002/cber.190503802176.
- ↑ Irma Goldberg (1906). "Ueber Phenylirungen bei Gegenwart von Kupfer als Katalysator". Berichte der deutschen chemischen Gesellschaft. 39 (2): 1691–1692. doi:10.1002/cber.19060390298.
- ↑ Ray Q. Brewster and Theodore Groening, "Ether, p-nitrophenyl phenyl", Organic Syntheses, Coll. Vol. 2, p.445 Online article
- ↑ Minireview Catalytic CC, CN, and CO Ullmann-Type Coupling Reactions Florian Monnier, Marc Taillefer Angewandte Chemie International Edition Volume 48 Issue 38, pp. 6954–6971, 2009 doi:10.1002/anie.200804497
- ↑ Copper-Catalyzed Formation of Carbon–Heteroatom and Carbon–Carbon Bonds, Buchwals, Stephen, L. et al. 2003 WO02/085838 Link
- ↑ C. F. H. Allen and G. H. W. McKee (1943). "Acridone". Org. Synth.; Coll. Vol., 2, p. 15
- ↑ H.B. Goodbrand; Nan-Xing Hu (1999). "Ligand-Accelerated Catalysis of the Ullmann Condensation: Application to Hole Conducting Triarylamines". Journal of Organic Chemistry. 64 (2): 670–674. doi:10.1021/jo981804o.
- ↑ Klapars, A.; Antilla, J. C.; Huang, X.; Buchwald, S. L. (2001). "A General and Efficient Copper Catalyst for the Amidation of Aryl Halides and the N-Arylation of Nitrogen Heterocycles". J. Am. Chem. Soc. (Communication). 123 (31): 7727–7729. doi:10.1021/ja016226z.
- ↑ Klapars, A.; Huang, X.; Buchwald, S. L. (2002). "A General and Efficient Copper Catalyst for the Amidation of Aryl Halides". J. Am. Chem. Soc. (Article). 124 (25): 7421–7428. doi:10.1021/ja0260465.
- ↑ Strieter, E. R.; Blackmond, D. G.; Buchwald, S. L. (2005). "The Role of Chelating Diamine Ligands in the Goldberg Reaction: A Kinetic Study on the Copper-Catalyzed Amidation of Aryl Iodides". J. Am. Chem. Soc. (Communication). 127 (12): 4120–4121. PMID 15783164. doi:10.1021/ja050120c.
- ↑ Jones, C. P.; Anderson, K. W.; Buchwald, S. L. (2007). "Sequential Cu-Catalyzed Amidation-Base-Mediated Camps Cyclization: A Two-Step Synthesis of 2-Aryl-4-quinolones from o-Halophenones". J. Org. Chem. 72 (21): 7968–7973. PMID 17850097. doi:10.1021/jo701384n.
- ↑ CCXLIV.—Replacement of halogen in orthobromo-benzoic acid William Robert Hardy Hurtley, J. Chem. Soc., 1929, 1870 doi:10.1039/JR9290001870