Jadomycin
 | |
| Names | |
|---|---|
| Other names
Jadomycin L-isoleucine | |
| Identifiers | |
| Properties | |
| C30H31NO9 | |
| Molar mass | 549.58 g·mol−1 |
| Appearance | Deep red-purple solid |
| Solubility | Soluble in various organic solvents |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
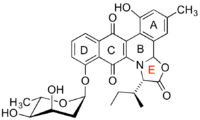
A jadomycin is an angucyclic natural product produced by Streptomyces venezuelae ISP5230 (ATCC10712[1]), the producing organism of the antibiotic chloramphenicol. The name jadomycin encompasses a family of related structures with variability about the E ring (usually an oxoazolone ring), which arises from the spontaneous incorporation of an amino acid during biosynthesis. Spontaneous reactions are uncommon in biosynthesis, but in the case of jadomycins this feature has been exploited towards the generation of jadomycin librairies based on different amino acids. Jadomycin A was the first compound of this family to be isolated and constitutes the angucylic backbone with L-isoleucine incorporated into the E-ring.[2] A related analog, jadomycin B, is modified by glycosylation with a 2,6-dideoxy sugar, L-digitoxose.[3] Jadomycins have cytotoxic and antibacterial properties.
Biosynthesis
The jadomycin biosynthetic gene cluster is well characterized. Jadomycin biosynthesis is based on type II polyketide synthase (T2Pks) assembly to generate the angucycline component,[4] and a dideoxy sugar pathway, generating the sugar donor NDP-L-digitoxose.[5] Studies have implicated JadG, an FAD-dependent oxygenase, in the ring cleavage required for incorporation of amino acids.[6] JadS, the glycosyltransferase that transfers L-digitoxose, has been shown to be flexible with respect to the sugar donor.[7][8][9]
Analogs based on E-ring modification
Jadomycin analogs have been obtained through culture of S. venezuelae in the presence of a single amino acid. The diversity of jadomycins includes those incorporating naturally occurring amino acids,[10] non-proteinogenic amino acids,[11][12] and synthetic amino acids with handles enabling further chemical modification.[13][14]
References
- ↑ "Streptomyces venezuelae Ehrlich et al. ATCC ® 10712™". www.atcc.org. Retrieved 2017-05-31.
- ↑ Ayer, Stephen W.; McInnes, A. Gavin; Thibault, Pierre; Walter, John A.; Doull, Janice L.; Parnell, Tracy; Vining, Leo C. (1991-10-28). "Jadomycin, a novel 8H-benz[b]oxazolo[3,2-f]phenanthridine antibiotic from streptomyces venezuelae ISP5230.". Tetrahedron Letters. 32 (44): 6301–6304. doi:10.1016/0040-4039(91)80152-V.
- ↑ DOULL, JANICE L.; AYER, STEPHEN W.; SINGH, AMRIT K.; THIBAULT, PIERRE (1993-05-25). "Production of a novel polyketide antibiotic, jadomycin B, by Streptomyces venezuelae following heat shock.". The Journal of Antibiotics. 46 (5): 869–871. ISSN 0021-8820. doi:10.7164/antibiotics.46.869.
- ↑ Han, Lei; Yang, Keqian; Ramalingam, Eswar; Mosher, Roy H.; Vining, Leo C. (1994). "Cloning and characterization of polyketide synthase genes for jadomycin B biosynthesis in Streptomyces venezuelae ISP5230". Microbiology. 140 (12): 3379–3389. doi:10.1099/13500872-140-12-3379.
- ↑ Wang, Liru; White, Robert L.; Vining, Leo C. (2002). "Biosynthesis of the dideoxysugar component of jadomycin B: genes in the jad cluster of Streptomyces venezuelae ISP5230 for l-digitoxose assembly and transfer to the angucycline aglycone". Microbiology. 148 (4): 1091–1103. doi:10.1099/00221287-148-4-1091.
- ↑ Tibrewal, Nidhi; Pahari, Pallab; Wang, Guojun; Kharel, Madan K.; Morris, Caleb; Downey, Theresa; Hou, Yanpeng; Bugni, Tim S.; Rohr, Jürgen (2012). "Baeyer–Villiger C–C Bond Cleavage Reaction in Gilvocarcin and Jadomycin Biosynthesis". Journal of the American Chemical Society. 134 (44): 18181–18184. PMC 3498853
 . PMID 23102024. doi:10.1021/ja3081154.
. PMID 23102024. doi:10.1021/ja3081154. - ↑ Jakeman, David L.; Borissow, Charles N.; Graham, Cathy L.; Timmons, Shannon C.; Reid, Taryn R.; Syvitski, Ray T. (2006-08-29). "Substrate flexibility of a 2,6-dideoxyglycosyltransferase". Chemical Communications (35): 3738. ISSN 1364-548X. doi:10.1039/B608847C.
- ↑ Forget, S. M.; Na, Jungwook; McCormick, N. E.; Jakeman, D. L. (2017-03-28). "Biosynthetic 4,6-dehydratase gene deletion: isolation of a glucosylated jadomycin natural product provides insight into the substrate specificity of glycosyltransferase JadS". Organic & Biomolecular Chemistry. 15 (13): 2725. ISSN 1477-0539. doi:10.1039/C7OB00259A.
- ↑ Li, Liyuan; Pan, Guohui; Zhu, Xifen; Fan, Keqiang; Gao, Wubin; Ai, Guomin; Ren, Jinwei; Shi, Mingxin; Olano, Carlos (2017-04-20). "Engineered jadomycin analogues with altered sugar moieties revealing JadS as a substrate flexible O-glycosyltransferase". Applied Microbiology and Biotechnology: 1–10. ISSN 0175-7598. doi:10.1007/s00253-017-8256-y.
- ↑ Jakeman, David L.; Farrell, Spring; Young, Wendy; Doucet, René J.; Timmons, Shannon C. (2005-03-01). "Novel jadomycins: incorporation of non-natural and natural amino acids". Bioorganic & Medicinal Chemistry Letters. 15 (5): 1447–1449. doi:10.1016/j.bmcl.2004.12.082.
- ↑ Jakeman, David L.; Graham, Cathy L.; Reid, Taryn R. (2005-12-01). "Novel and expanded jadomycins incorporating non-proteogenic amino acids". Bioorganic & Medicinal Chemistry Letters. 15 (23): 5280–5283. doi:10.1016/j.bmcl.2005.08.047.
- ↑ Forget, Stephanie M.; Robertson, Andrew W.; Overy, David P.; Kerr, Russell G.; Jakeman, David L. (2017-05-18). "Furan and Lactam Jadomycin Biosynthetic Congeners Isolated from Streptomyces venezuelae ISP5230 Cultured with Nε-Trifluoroacetyl-l-lysine". Journal of Natural Products. ISSN 0163-3864. doi:10.1021/acs.jnatprod.7b00152.
- ↑ Dupuis, Stephanie N.; Robertson, Andrew W.; Veinot, Thomas; Monro, Susan M. A.; Douglas, Susan E.; Syvitski, Ray T.; Goralski, Kerry B.; McFarland, Sherri A.; Jakeman, David L. (2012-04-02). "Synthetic diversification of natural products: semi-synthesis and evaluation of triazole jadomycins". Chemical Science. 3 (5): 1640. ISSN 2041-6539. doi:10.1039/C2SC00663D.
- ↑ Robertson, Andrew W.; Martinez-Farina, Camilo F.; Smithen, Deborah A.; Yin, Huimin; Monro, Susan; Thompson, Alison; McFarland, Sherri A.; Syvitski, Raymond T.; Jakeman, David L. (2015). "Eight-Membered Ring-Containing Jadomycins: Implications for Non-enzymatic Natural Products Biosynthesis". Journal of the American Chemical Society. 137 (9): 3271–3275. doi:10.1021/ja5114672.