Isoxanthohumol
 | |
| Names | |
|---|---|
| IUPAC name
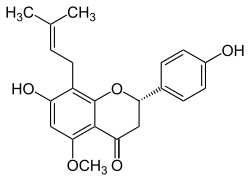
7-hydroxy-2-(4-hydroxyphenyl)-5-methoxy-8-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| PubChem CID |
|
| |
| |
| Properties | |
| C21H22O5 | |
| Molar mass | 354.39 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isoxanthohumol is a prenylflavonoid, and it is a phytoestrogen. It is abbreviated as IX or IXN.
8-Prenylnaringenin can be produced from isoxanthohumol by flora in the human intestine,[1] and by fungi in cell cultures.[2]
This prenylflavonoid is found in hops and beer. It has limited estrogenic activity. At the concentration found in beer, it is unlikely to have an estrogenic effect in breast tissue.[3]
Derivatives of isoxanthohumol are: 7,4′-Di-O-methylisoxanthohumol; 7-O-methylisoxanthohumol; 7-O-n-pentylisoxanthohumol; 7,4′-di-O-n-pentyl-8-isoxanthohumol; 7,4′-Di-O-allylisoxanthohumol; 7,4′-Di-O-acetylisoxanthohumol; and 7,4′-Di-O-palmitoylisoxanthohumol.[4]
See also
- Xanthohumol, the corresponding prenylated chalcone
References
- ↑ Possemiers S, Bolca S, Grootaert C, Heyerick A, Decroos K, Dhooge W, De Keukeleire D, Rabot S, Verstraete W, Van de Wiele T, et al. (2006). "The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine". Journal of Nutrition. 136 (7): 1862–7. PMID 16772450.
- ↑ Fu, Ming-Liang; Wang, Wei; Chen, Feng; Dong, Ya-Chen; Liu, Xiao-jie; Ni, Hui; Chen, Qi-he (2011). "Production of 8-Prenylnaringenin from Isoxanthohumol through Biotransformation by Fungi Cells". Journal of Agricultural and Food Chemistry. 59 (13): 7419–26. PMID 21634799. doi:10.1021/jf2011722.
- ↑ Bolca, Selin; Li, Jinghu; Nikolic, Dejan; Roche, Nathalie; Blondeel, Phillip; Possemiers, Sam; De Keukeleire, Denis; Bracke, Marc; Heyerick, Arne; Van Breemen, Richard B.; Depypere, Herman (2010). "Disposition of hop prenylflavonoids in human breast tissue". Molecular Nutrition & Food Research. 54: S284–94. PMC 3856213
 . PMID 20486208. doi:10.1002/mnfr.200900519.
. PMID 20486208. doi:10.1002/mnfr.200900519. - ↑ Anioł M, Swiderska A, Stompor M, Zołnierczyk AK (2012). "Antiproliferative activity and synthesis of 8-prenylnaringenin derivatives by demethylation of 7-O- and 4'-O-substituted isoxanthohumols". Med Chem Res. 21 (12): 4230–4238. PMC 3474914
 . PMID 23087590. doi:10.1007/s00044-011-9967-8.
. PMID 23087590. doi:10.1007/s00044-011-9967-8.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.